Physicist: The short answer is; you probably can, but not for long.
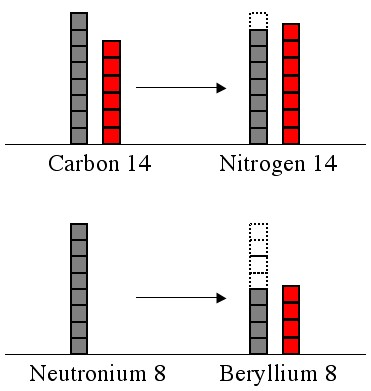
If you’ve taken a little chemistry you probably know that the electrons in an atom “stack up” in energy levels. The more electrons you add, the higher the electrons have to stack. The same is true for the protons and neutrons inside the nucleus of the atom. What’s a little surprising is that the stack for the protons and the stack for the neutrons are independent of each other.
In a stable atom the energy in the proton stack will be about the same as the energy in the neutron stack. If they’re unequal, then a neutron will turn into a proton ( decay), or a proton will turn into a neutron (
decay) to even out the levels. The greater the difference the sooner the decay, and the more radioactive the atom. There are plenty of exceptions (e.g., Uranium 237 vs. 238), but the pattern usually holds.
An atomic nucleus made entirely out of neutrons (known to some sci-fi aficionados as “neutronium”) would be completely imbalanced and would decay instantly. It would be tremendously radioactive.
Chemistry nerds may have noticed that heavier elements have more neutrons than protons. For example, Iron 58 has 26 protons, 32 neutrons, and is stable. Forcing protons together takes a lot of energy (likes repel), so after hydrogen, proton energy levels get higher, quicker, than than neutron energy levels.
Exception! In neutron stars you have the added component of lots of gravity. When a proton and an electron fuse into a neutron they take up less room, and since gravity wants to crush everything together, this is a lower energy state. So neutron stars are the one example of stable neutronium. However, most people would say that calling an ex-star an atomic nucleus is pushing the definition of “atomic” a bit far. Even more exciting; neutron stars may also contain the only naturally occurring stable lambda particles!
The picture of the completely ordinary chemist is from here.





Pingback: Q: What are Feynman diagrams, how are they used (theoretically&practically), and are there alternative/competing diagrams to Feynman’s? « Ask a Mathematician / Ask a Physicist
This brings up a question: Given that protons have an incredibly long lifetime (potentially longer than the so-far age of the universe, according to Leonard Susskind, and that their partners in nucleon-hood have a wee short lifetime of about 15 minutes, how are any atoms stable? Every 15 minutes a proton could have to make up for a neutron loss and become a neutron (…”If they’re unequal, then a neutron will turn into a proton , or a proton will turn into a neutron to even out the levels.”) An atom could become that of an altogether different element roughly every 15 minutes. As this clearly doesn’t happen, what am I missing?
If you look at the beryllium 8 example above, in order for a neutron to become a proton you’d need to add energy. If that energy isn’t available, then the neutrons in the atom are stable.
Perhaps my original premise was mistaken or unclear. I had read in a number of places that the average lifespan of a neutron is about fifteen minutes. If this is wrong, my question is meaningless. Are you saying that in a stable atom the average lifespan of a neutron is about as long as that of a proton? Thanks for your patience.
You’re very clear!
The half life of an isolated neutron is about 15 minutes, but that changes with the neutron’s circumstances. Specifically, being in an atom means that the neutron’s half life can be made much shorter, or much, much longer.
In the context of this post, you can think of a neutron as an atom with 1 neutron and 0 protons. By decaying into a proton, the proton and neutron stacks come slightly closer to being in balance.
In a stable atom the neutrons are perfectly happy to exist for an arbitrarily long time.
Many thanks for clearing that up. This site is incredibly helpful.
Pingback: Q: What is radioactivity and why is it sometimes dangerous? | Ask a Mathematician / Ask a Physicist
Beryllium 8 falls apart into 2 helium 4’s.
would it be possible to find a certain number of neutrons that would crate a stable neutronium material, if you didn’t add energy for them to become protons? this material should have incredible properties, it should be extremely dense, as an atom’s size is 10^11 metres across, and a nucleus’ size is 10^15 metres across, so an atom is 10,000 times wider than a neutron. this means you could fit 10^12(1,000,000,000,000) neutrons into the space of an atom. this has crazy implications. this material in the space of a 1kg weight would weigh 10^12 kg. also if you were to compress the earth using this(not that you practically could) it would go from 6,371,000 metres across, to only 637.1 metres across. it would probably behave like an extremely fine dust, so it would probably have to be contained in something. if you fired it through the air, it would have a lot of momentum and inertia, but almost no air resistance.
In reference to your comment of November 12, 2011 at 1:50 am:
The half-life of an isolated neutron is about 10 minutes. It is the mean lifetime that is about 15 minutes.
Please reply
What would happen if an atom of neutronium had an odd amount of neutrons?
Would the one remaining neutron be converted to beta particles and photons,etc?
@Ryan
It depends on the total number and the violence of the reaction (the nucleus may just fall apart). Very large nuclei have many more neutrons than protons.
@The Physicist
What about an atom of neutronium 9 was suspended in a perfect vacuum, and nothing interferes with the atom? What would happen then?
Sir please consider,
What decides the Half Life duration of the particles??
If the lifespan of a neutron is only 15 minutes, how does neutron stars exist?
The lifespan of a neutron is not 15 minutes. That time period is the _average_ lifetime of a free neutron. The half-life of a free neutron is 10 minutes, meaning that if you wait 10 minutes starting at any time, close to one-half of the neutrons will decay. So they never go away completely.
Now, neutrons in a neutron star do not have energy levels that allow for beta decay. IOW, if one of the neutrons would beta decay (turn into a proton, electron, and an anti-neutrino) in a neutron star, the energy of new state of the star plus the energy of the electron and antineutrino would be higher! Thus it is not allowed. You’d have to supply some energy to make it happen.
Think of a rock on one of the steps of a staircase. You need to give it a kick for it to fall. Better yet, imagine each step has a well, and the rock is at the bottom of the well. You need to supply energy for the rock to get out and fall to the next step. A free rock will fall by itself.
TL;DR, they kind of exist