Physicist: Lord Kelvin (and others of his ilk) noticed that when you hold the volume of an ideal gas constant you get a nice, linear relationship between pressure and temperature.
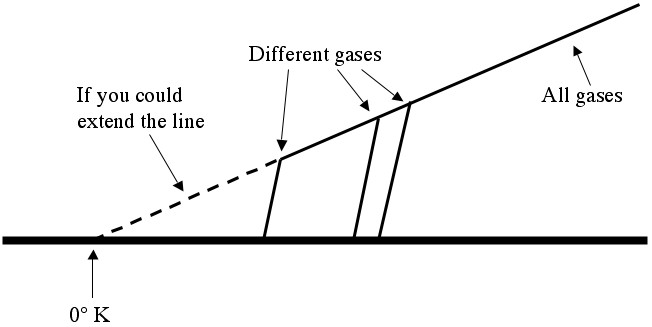
Temperature vs. Pressure. PV = nRT works for all ideal gases, independent of the particular gas in question (helium, water vapor, sulfur hexafluoride, ...). Different gases become liquids or solids (and stop exerting pressure) at different temperatures, so they drop off of the "ideal gas graph" at different points.
By the by, an ideal gas is just a gas where you can assume that the particles are bouncing off of each other much harder than they’re trying to stick together (the gas is hot enough that it’s a long way from condensing). This assumption allows you to use the “billiard ball model” of gas dynamics, which in turn leads to the ideal gas law (), which says that you should expect a nice straight line (like the one above).
Although it’s impossible to cool anything off completely, and despite the fact that all of the gases that Lord Kelvin was working with became liquids when chilled enough, it was still easy to graph temperature vs. pressure (even around room temperature) and then extend the line to find the temperature where the pressure should be zero. Kelvin figured that this would be a much more natural place for “zero” to be, and he carefully measured it (by extending the line) to be around -273.15°C, which is now 0°K (zero degrees Kelvin).
Using Kelvins instead of Celsius means that you can bust out the ideal gas law without needing to adjust anything. If you wrote the ideal gas law using Celsius instead, it would be , which is ugly.







14 Responses to Q: How did Lord Kelvin come up with the absolute temperature? I mean, how could he say surely that it was 273.15 C below zero?