Physicist: The magnetic properties of a material are governed entirely by the configuration of the electrons in that material. In metals there are two types of electrons: bound electrons and free electrons. The free electrons are free to move between atoms, and are the cause of conductivity in metals. The bound electrons are stuck to the individual atoms.
Each electron, in addition to having charge, also has a “magnetic moment” which is a fancy way of saying that it’s a tiny bar magnet. Generally the bound electrons will be paired off in opposite spin pairs. This is like putting a North-South magnet next to a South-North magnet. They almost completely cancel each other out. However, sometimes (in iron, nickel, and cobalt for example) you’ll have one or more un-paired electrons. The magnetic fields of these electrons aren’t canceled out by another, oppositely-oriented, electron. As such they lend an overall magnetic field to the atom they inhabit.
So, some metals are attracted to magnets because they are full of tinier magnets. Those tinier magnets twist about so that they align with the field of the larger magnet. However, that just pushes the question back to “Why do magnets attract each other?”.
Those free electrons aren’t completely useless. If they’re exposed to a changing magnetic field (wave your magnet around) they’ll start moving around in “eddy currents”. Those eddy currents always try to resist the changing field (“Lenz’s law” or “the universe is a stubborn jerk law”). So all conductive metals interact with magnetic fields (otherwise generators wouldn’t work), but not in the “attracted to” kind of way.
Answer gravy: “Why do magnets attract each other?” Magnetic fields, like high school students, don’t really want to exist. A magnetic field of strength B that fills up a volume V has an associated energy . So creating magnetic fields takes energy, and getting rid of them frees up energy.
It turns out that processes that release energy are usually forces. For example; when you drop an object energy is released, and it so happens that gravity is a force. Similarly, magnets will try to line up in such a way that they will cancel out each other’s fields. Less fields = less energy. So, the process of lining up to cancel out their fields decreases the energy tied up in those fields, and as such there’s a force that tries to line up the magnets.
Also: The physics behind the magnetic properties is really nasty. Nasty enough that the math can’t be done, and computer simulations can’t be trusted (generally). Here’s a map of the (experimentally found) magnetic properties on the periodic table:

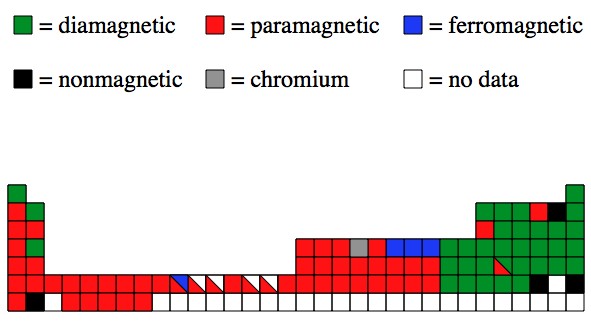






29 Responses to Q: What causes iron, nickel, and cobalt to be attracted to magnets, but not other metals?