Physicist: Frequently in quantum mechanics you’ll find that particles are restricted to only a certain set of states or locations, and yet somehow they can move from one to the next. It’s like moving between islands without crossing any water.
“Classically” (19th century, pre-relativity, pre-quantum) this is impossible. If you see a particle in one place and then see it again, but in a new place, then of course it must have traversed the distance from one to the other.
But here’s the essential difference between quantum mechanics (correct) and classical physics (wrong): particles aren’t solid objects that have a genuine position, instead they’re waves that are “smeared out”.
A standing wave (like a guitar string, or an electron orbital) usually has “nodes” where the wave is always zero.
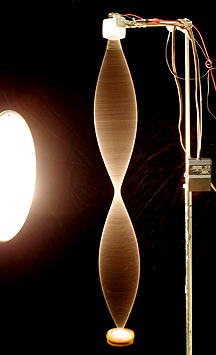
The string remains stationary in the middle, but the wave has no problem getting past that point. This image stolen, unrepentantly, from http://people.rit.edu/andpph/text-string-vibrations.html.
But of course there’s a big difference between the wave being zero at a node, and the wave being unable to get to the other side of that node. It’s the difference between the string in the above picture, and what you would have if you nailed that string to a piece of wood at the node and left the bottom half dangling.
For you calculus buffs out there, the difference is hidden away in the derivatives.
If you have a particle (wave) like this and you measure where it is many times you’ll find that it’s on the top about half the time, on the bottom about half the time, and never ever in the middle (ever).
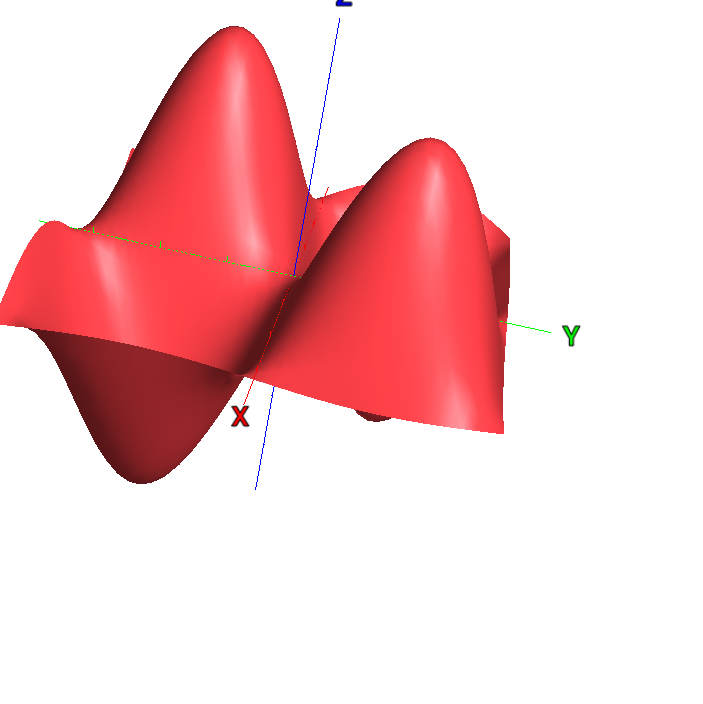
The shape of the standing waves formed inside a microwave oven. The peaks and troughs move up and down, but the lines in between are stationary and zero. This is why microwaves have rotating plates, to keep food from sitting in the nodes (and staying cold) or from staying in the peak areas (and burning).
One of my personal fave examples of “large scale” quantum weirdness is microwave ovens. A microwave oven creates a standing wave of photons with a “plus-shaped” (+) node, which leaves the center of the chamber especially cold. The chance of finding a photon anywhere on the plus-shaped-node is zero. So there are four different cells that it should be impossible for the microwave photons to move between, but they don’t seem to mind at all. It’s not like particles exist or something.
So thinking of things like electrons as particles leads to incorrect conclusions. Thinking of them as waves is really the way to go.







Pingback: Quantum Physics May Upend Our Macroscopic Reality In The Universe - Science and Tech News