The original question was: With the current technology, it is possible now to transmute lead into gold, or whatever element into another? What transmutations should have tried the ancient Alchemist instead of the famous lead-gold one, in order to find an easy and useful success?
Physicist: Lead to gold: no. But you can change some elements into others. The yield is famously tiny, and the process is prohibitively expensive. Before the late 19th century, no body had ever observed one element turning into another, and until the 20th century there was no equipment on Earth that had the faintest prayer of successfully changing one element into another (on purpose).
Back in the day, when chemists (alchemists) were getting good at purifying samples and making fancy chemicals, they got pretty cocky about turning stuff into other stuff. But while you can use basic chemical reactions to turn hydrogen and oxygen into water, or flour and water into bread, there’s no combination of chemicals and reactions that even start to change one element into another. Alchemists back in the day, being unaware of these sorts of things, got very excited about lead-to-gold stuff, philosopher’s stones, and life from nothing. Many of them were legit scientists of the day, so we legit scientists of today have inherited a lot of their symbols and short-hand (though not their methods, by and large).

Newton loved himself some alchemy. Sure he did calculus and science, but he also did pioneering work into finding the holy grail and even a variety of crazy pursuits.
Fancy chemicals and molecules are different because they use different combinations of elements, but elements and isotopes are different from each other because they have different numbers of protons and neutrons in their nuclei.
There are basically three ways to change the number of protons and neutrons in the nucleus of an atom. Fusion, radioactive decay, and neutron bombardment.
There are some issues with practical fusion. To date we’ve managed to fuse deuterium (hydrogen) into helium, which is the easiest fusion there is, even then just barely, and only to useful effect in the middle of very big bombs. To use fusion to make gold (which is the way gold is created in nature) you need a super nova, which would probably be expensive. Also impossible.
A lot of atoms have unstable nuclei that will occasionally “pop” and turn into another element or isotope. So, technically, being patient is one way to turn a sample of some material into another.
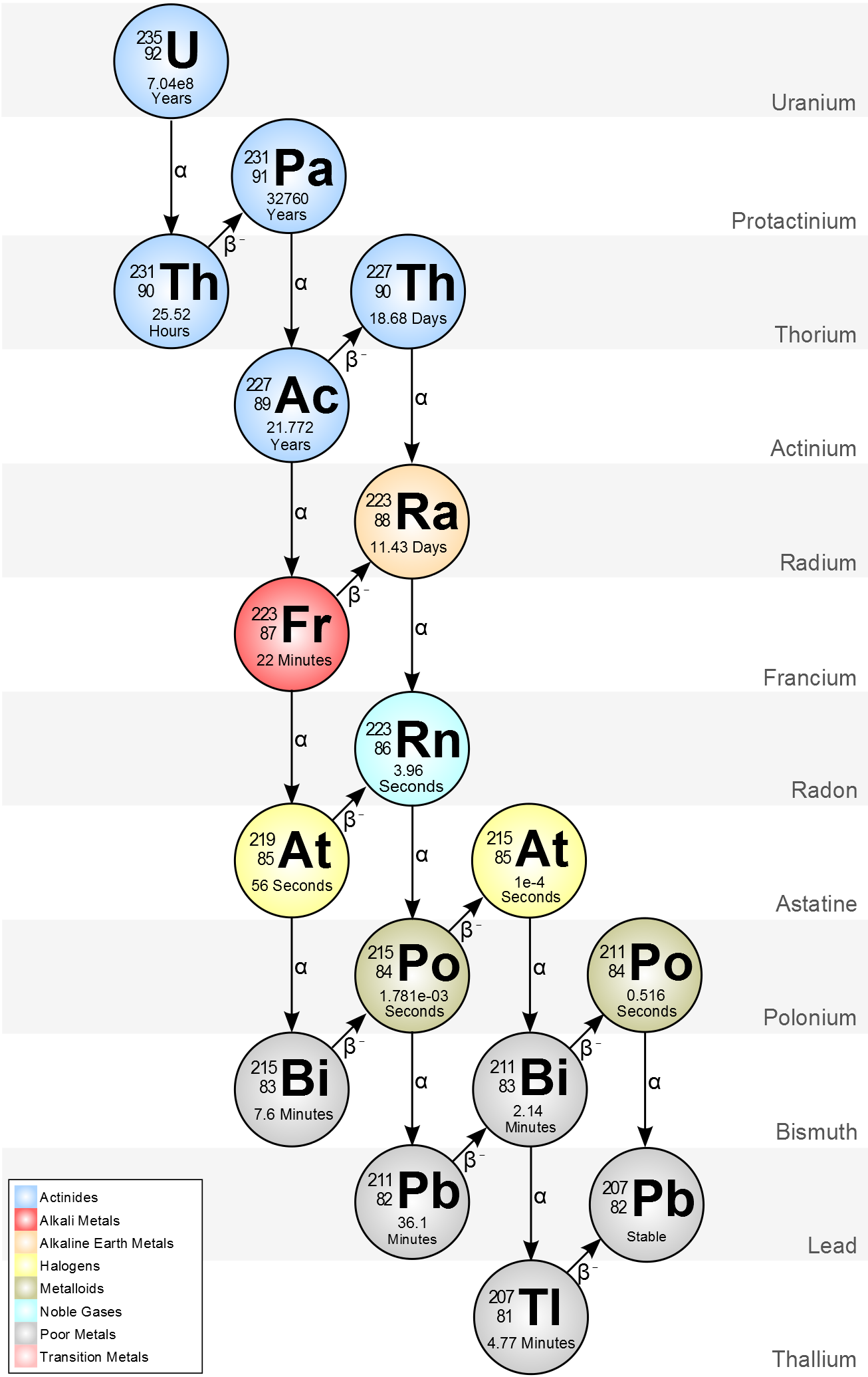
Start with some Uranium 235, then wait for several hundred million years, and you’ve got mostly lead.
Unfortunately, the material in question has to be radioactive beforehand. Radioactive atoms “decay” until the number of protons and neutrons are in balance (not equal, just balanced in a particular way), and for heavy elements that balance is almost always reached with lead or thallium.
The last way to change one isotope to another, and the only technique we can really use and control, is “neutron bombardment“. Neutron bombardment isn’t the best option, so much as it’s the only option. The idea is that since neutrons are electrically neutral (hence the name) they can enter and join the nucleus of an atom without being repelled by the positively charged nucleus (this repulsion is why this technique doesn’t work with protons, and why fusion in general is so difficult).
Bombardment is how plutonium is manufactured from uranium. Bombarding a sample with neutrons sometimes makes the atoms in question decay into higher elements, and almost always makes them more radioactive (so this is a “bombard then wait” sort of thing). In some cases it makes them so spectacularly radioactive that they immediately fly apart, and if they also produce a spray of neutrons, then you’ve got yourself the makings of a bomb or a power plant.
Here’s a map of all of the known isotopes and their preferred means of decay (many isotopes have several ways they can decay). The full chart, in detail, can be found here. It’s a very big picture.
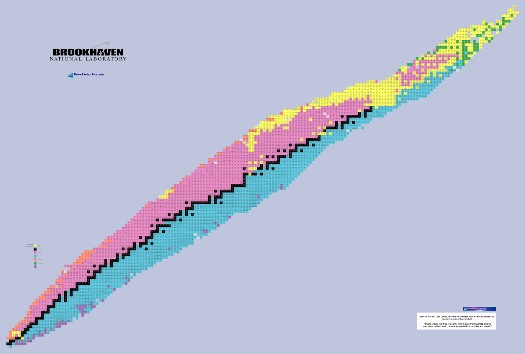
All of the isotopes, with the number of neutrons increasing as you go to the right, and the number of protons (which is the “atomic number” or “element number”) increasing as you go up. The black squares are the stable isotopes, and this region is called the “valley of stability”.
The different colors indicate different decay paths. For example, pink is β+ decay, which turns a proton into a neutron, and an extra anti-electron, which in this case is the “radiation” we detect flying out. So, on the chart the pink isotopes decay down and to the right, by losing one proton and gaining one neutron.
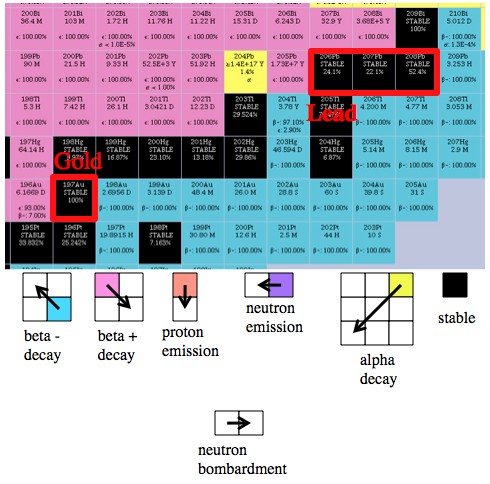
How to “play the game”. At best, we can cause a tiny fraction of a sample of an isotope to move one to the right (gain one neutron).
By looking at the chart you can figure out what elements can reasonably be made from others using neutron bombardment. For example, you might look at this little part of the chart (picture above) and think that you should be able to create gold by bombarding platinum 196 (which is directly below gold 197, the only stable gold isotope). This would add a neutron, which changes some of the sample into platinum 197, which would then execute a β– decay, moving up and to the left, and turn into gold. As it happens, this is exactly how you create gold from platinum. That β– decay has a half-life of about 20 hours, so once you irradiate your platinum you only have to wait a few days before extracting the trace amounts of gold from your sample.
There’s also an isotope of mercury, mercury 196, that can be turned into gold (it’s above and two to the left from gold 197).Lead, on the other hand, is in a bad position to form gold. Using neutron bombardment you can move to the right on the chart, but if you follow the decay path from every heavy isotope of lead, they all lead back to either lead or bismuth.
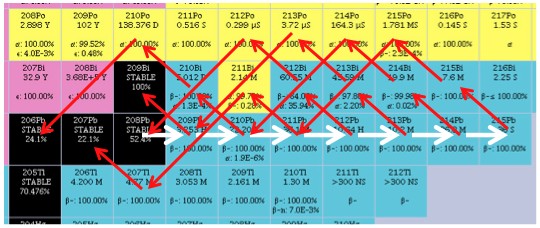
All we can do is add neutrons (white arrows), but that just takes puts us on “decay paths” that lead back to lead or bismuth.
So, using the one and only technique available to us, we definitely cannot turn lead into gold. Not even a little bit. Platinum and one fairly rare isotope of mercury, sure. But not lead.
Also, both the platinum and mercury processes are substantially more expensive and dangerous than digging gold out of the ground. Among other things you need to get your hands on a neutron source, which is generally an extremely radioactive (illegal and expensive) metal, or a multi-billion dollar accelerator used to blow apart heavy isotopes into buckets of neutrons. There are easier ways to lose money.








Pingback: Comment transformer le plomb en or ? | Pourquoi Comment Combien
Pingback: Comment transformer le plomb en or ? | Just Science | Prenez donc un petit nuage de Science
Pingback: Comment transformer le plomb en or ? | Pourquoi Comment Combien