Physicist: Just so we can talk about this using physics rather than poetry, for the sake of this article “color” really means “frequency”. Light frequency is a bit more objective than color and includes things we can’t see (like ultraviolet).
When you put gas in a tube and pass electricity through it you get light. Electro-dynamically speaking, this is basically just beating the hell out of the atoms and letting the atoms ring like bells (only emitting light instead of sound). Individual atoms are like simply shaped bells; the “tones” they make (or absorb) are very specific. The colors emitted by atoms, their “spectra”, are different for different elements. This is tremendously useful because it allows us to look at the light coming from something and immediately know what that thing is made of.
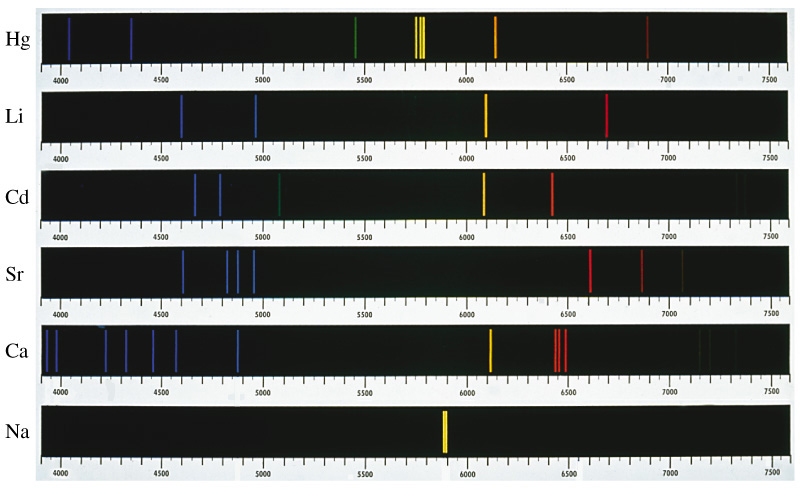
When given the energy (like in a neon light) atoms will emit light with very specific frequencies. These are the lines for mercury, lithium, cadmium, strontium, calcium, and sodium that happen to fall in the visual spectrum. There are many more lines that we mere humans can’t see.
Some colors fall into the gaps between the spectral lines of all elements (technically, almost all of them do). So you can be forgiven for thinking that there are some colors that just never show up in nature. Fortunately, there are a lot of effects that shift all those lines, blur them, or even split them.
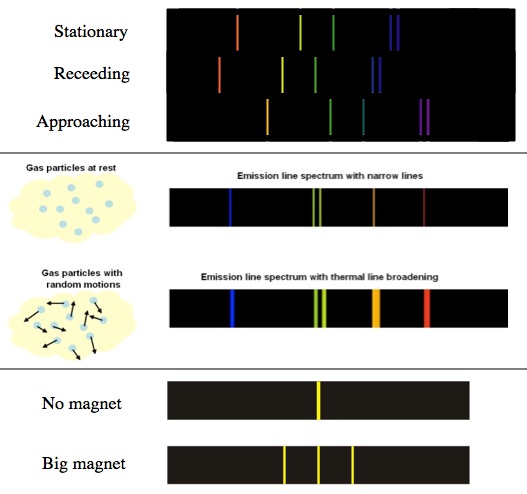
Top: When a light source moves toward or away from you its spectrum is shifted up or down. Middle: In a hot gas the atoms are moving randomly, so whether the lines are Doppler shifted up or down is also random. This broadens the spectral lines. Bottom: With a very strong magnet you can change some of the electron’s energy levels and as a result transitions that would normally create the same color become separated.
So you can create any color by starting with a few distinct colors and then moving your light source either toward or away from an observer to Doppler shift one of your colors to the target color. That’s a little like using a piano to get some notes, and then driving it around to get all the notes in-between.

An efficient means to play C above high C.
You can also just use a non-atomic source of light, like something that’s glowing hot, and then select out the color you want with a monochromator (the rest is chucked out). But, as with any process that involves throwing out almost everything, this is remarkably inefficient.
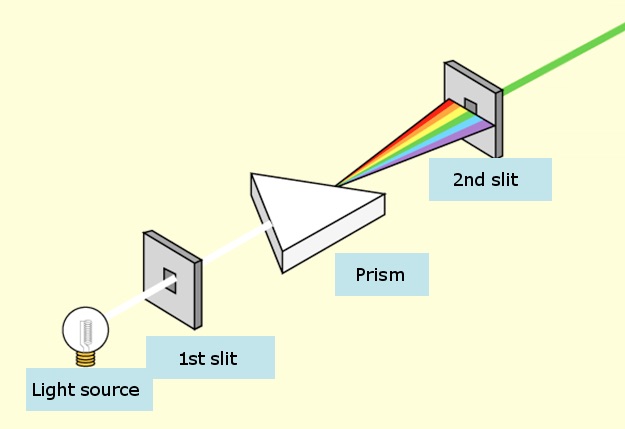
Monocromators generate light with a single color (one might say “mono-chromatic” light) by just throwing away all the light that isn’t the right color.
So, say you want to create a very specific color of light with as little “waste light” as possible. Well, a good place to start is lasers. For some slick quantum reasons, the photons in laser beams are all kinda “clones” of each other; inside of any kind of laser device, the presence of the right kind of photon encourages the creation of other identical photons. Pretty soon your laser is bubbling over with coherent, identical photons and not a lot else. These share, among other things, a common color.
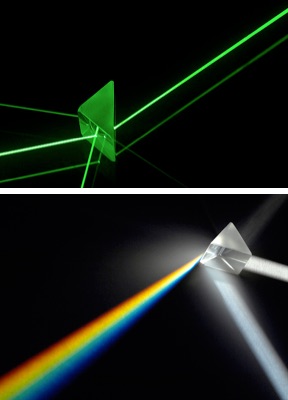
Laser beams: not a lot of colors.
It turns out that only a very small fraction of atomic spectral lines are good candidates for lasers. It is possible to create laser light at any frequency between microwaves and X-rays, but the technique is a long way from efficient. You can use the Doppler effect to change the color of your laser, but in order to make any significant change you’ll need to get it going a significant fraction of the speed of light.
If you want to efficiently create any very specific color of light, you just need to strap a laser to a starship. So… no need to be picky.

An efficient means to make green light.







11 Responses to Q: Are some colors of light impossible? Can any color of light be made?