The complete question was: Is it true that all matter is simply condensed energy? Does that mean that the Big Bang was pure energy and coalesced into matter?
Physicist: Pretty much. If you can get enough energy into one place (generally light or kinetic energy), then you’ll get a (mostly random) variety of particles popping out. The conversion between mass and energy is so ubiquitous in physics, that most physicists only know the mass of particles in the context of their equivalent energy. If you ask a physicist “what is the mass of an electron?” they’ll say “0.5 MeV” (which is a unit of energy). Frankly, it’s more important to know than the actual mass. I mean, how hard is it to pick up an electron? If you answered “I don’t care” or “zero”, you’re right.
The only thing that keeps particles from turning back into energy (again, usually light and kinetic) are “conserved quantities”. If you’ve taken an intro physics course you should be familiar with conservation of energy and momentum. In particle physics you also need things like: charge, Lepton flavor (which covers things like electrons and neutrinos), and Baryon number (which covers things like protons and neutrons).
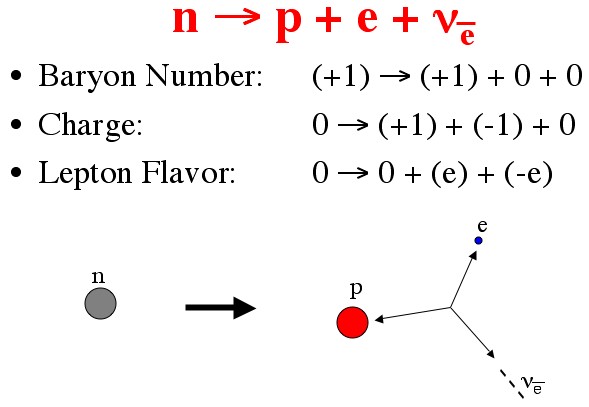
The classic example is neutron decay:
A neutron is heavier than a proton, so you’d think it would decay into a proton and some extra energy (conserving energy and baryon number). But that would violate conservation of charge (protons have 1, neutrons have zero). So maybe it could decay into a proton and electron? Now you’ve balanced charge, but violated lepton flavor (electrons have “electron flavor 1”). To balance everything you need to add an anti-electron neutrino (electron flavor -1) to the mix.
The very early universe was a “particle soup”. The mean energy of the photons flying about was more than enough to generate new particles. These would pop into existence in balanced quantities and then cancel out again. The big difference between now and then (why we don’t see particles being spawned off all the time) is that the average energy of photons today is closer to 660 meV (about 1 billionth of the energy needed to create electrons, the smallest particle).







Informative and funny.
Pingback: Q: What are Feynman diagrams, how are they used (theoretically&practically), and are there alternative/competing diagrams to Feynman’s? « Ask a Mathematician / Ask a Physicist
In your response, your last paragraph raised a question for me, a non-physicist. It follows: “The very early universe was a “particle soup”. The mean energy of the photons flying about was more than enough to generate new particles. These would pop into existence in balanced quantities and then cancel out again. The big difference between now and then (why we don’t see particles being spawned off all the time) is that the average energy of photons today is closer to 660 meV (about 1 billionth of the energy needed to create electrons, the smallest particle).” My question regards the energy loss mentioned in the final sentence. Since energy must be conserved, is that lost energy the “glue” which is holding together the matter which exists after the “big bang”? If not, what did that energy convert to? What is it’s present nature? If matter is energy, energy composes matter, and there is nothing else in the equation, what else can it be?
My question regards the energy loss mentioned in the final sentence. Since energy must be conserved, is that lost energy the “glue” which is holding together the matter which exists after the “big bang”? If not, what did that energy convert to? What is it’s present nature? If matter is energy, energy composes matter, and there is nothing else in the equation, what else can it be?
The continous expansion of the universe has the effect that the same amount of energy occupies a still larger volume, thus lowering the average energy density.
If we say that all is made up of energy then why there are different forms with same energy . e.g Humans , Animals , Plans are all made up of energy then due to which factor they have different forms.
I have a question about “The very early universe was a “particle soup”. The mean energy of the photons flying about was more than enough to generate new particles. These would pop into existence in balanced quantities and then cancel out again. The big difference between now and then (why we don’t see particles being spawned off all the time) is that the average energy of photons today is closer to 660 meV (about 1 billionth of the energy needed to create electrons, the smallest particle).”
What kind of particles can be produced? Can we see it by the naked eye?
Electrons would be the first to be created. They wouldn’t be visible on their own, but they would scatter light. That would make the area “foggy”, in addition to being flooded with high-energy radiation.
If we say that all is made up of energy then why there are different forms with same energy . e.g Humans , Animals ,….. are all made up of energy then due to which factor they have different forms.
Wouldn’t the one and only fundamental particle be some amount of energy, then?
T. Reid: Also a non-physicist here. My opinion is that the amount and character of matter that arose at the Big Bang are governed by equilibrium, similarly to dynamical systems and chemical reactions. This may or may not represent the views of particle physicists. It’s often difficult to tell exactly what they’re saying.
That’s an equilibrium between “energy” and “matter.”
it is a good question that how can a same energy can produce different types of masses,,,but looking at the atomic level we can find that the constituent unit of every matter is similar …a profound difference is produced between them is just because of the different atomic arrangements ,,,may be these differences rises with the existance of different energy density at a point……more amazingly the fact is our universe constitute of mass and energy and rest of the portion is empty and interconversion goes on between mass and energy according to the famous equation E=mc^2 ,,…
“If we say that all is made up of energy then why there are different forms with same energy . e.g Humans , Animals ,….. are all made up of energy then due to which factor they have different forms.”
if you look hard enough the only things that cannot be distinguished is the energy and fundamental particles themselves. their arrangement, at such tiny scales, forms progressively larger and larger structures on the allowable laws of their interactions (conservation laws, quantized energy levels, fine structure constant, etc).
animals will have some things in common (made up of cells) and other things that are not in common (some have hair, some are larger than others). but if you examine what you assume to be in common (made up of cells) you will find further ways to distinguish them – the cells of different animals vary. you can repeat this process down to the plank volumes making up everything if you wanted to but the only way to keep track at smaller and smaller scales is how you arrived there, or working backwards, every structural choice of the smallest, gives new possibilities for the larger scale.
Physicist: Pretty much. If you can get enough energy into one place (generally light or kinetic energy), then you’ll get a (mostly random) variety of particles popping out.
People have a property which is called “height”.
Can we say that people are condensed height?
If we get enough height into one place then will we get a (mostly random) variety of people popping out?
I think energy is same it is the DNA which make changes in that . DNA of the live act as Operating System which in terns has it’s own program to develop things from this energy . Other than living things all other things like stone ,… don’t have any program define in them so these things have rough shapes without any properties.
Question is now how to develop this DNA 🙂 with program to consume this energy .
Where did the original “energy” come from? Seems theory can try and explain why some things seems to happen (although continuously being “revised”), but the starting point still seems unexplained
My guess is we aren’t really close enough for anything but wild speculation, philosophy, and/or fantasy.
Hi Kevin, thanks for the response. What seems to fit available data the best – even if it is philosophic rather than a pure naturalistic explanation?
“People have a property which is called “height”.
Can we say that people are condensed height?
If we get enough height into one place then will we get a (mostly random) variety of people popping out?”
To Jon. As I understand it, height is like time, a concept and not an actual force or entity.
All elements are made up of same energy. As we know that energy is made up of vibrations and because of this we have different shapes and forms of matters. These are vibrating at different frequencies.
For more information check: Behavior of GOD partials at different vibration frequencies.
Ash: As I understand it, height is like time, a concept and not an actual force or entity.
I think Jon’s question was a rhetorical one. I understand him to have been equating energy to height – both are a property of matter, that cannot exist without said matter. So, he was asking how you can condense a bunch of it into one place in the absence of the substance of which it is a property?
I have the same question. Isn’t energy just a property of matter – the movement of matter, specifically? It seems to me like energy can’t exist without matter and matter can’t exist without energy… Chicken and egg.
To Dan, Jon. I understand it as matter that is a property of energy. Energy in a certain state and configuration that is matter. That matter is configured into all the things we see. Just my understanding.
Everything, of this univers is related to energy. E.g. Stars, space, gases, galaxies, planets, black hole etc everything, produce energy from matter or other resources, some uses itself, and some let it for others use, the storage OF energy increases the body Of mass, when we burns that same matter it repays its energy which it stored. In this era matter IS changed into energy. Can we covert same energy into matter?
Hi All, Conclusion of this topic is : Neither people from past 1M years get answer to this and nor ppl today/tomorrow will get this answer. So leave this topic and Enjoy the life .
I like Obama’s answer on Alien question when he ask is there is life on other planets he said “I don’t know is there is life out there on any other planet , but there is life on earth and we need to take care of this”. So instead of wasting your time Enjoy the life :). from all above discussion I come to know that people are with half knowledge with is dangerous 🙂 Everybody is trying to say I am something and looking of Visa in USA ha ha ha 🙂
my question is where are electrons and protons getting their energy from. Such as light which is made of different wave lengths of energy. Where does the energy come from and how long will it last? If we are seeing galaxies billions of light years away… shouldn’t the energy from the source degrade at some point in time and not continue endlessly.
No one can explain a dream , It is the seat of life and Intelligence, for a dream is it`s own master, this master has no explainable existence ,it is a Deity unto it`s own , this view we have of our awareness is a part of this dream it is beyond our knowledge and is unknowable to the thinking mind, the thinking mind cannot comprehend it`s own existence but can entertain the possibilities that it can do so, the Energy does not know where it came from it just knows it is there , I AM WHO I AM !
If you say everything is made of energy the universe is a very strange place indeed.
But if you say everything is made by energy, after all the description of energy is “the ability to do work” then everything is much simpler. Think of wave particle duality where the wave is the energy and the particle as the work being done, then space time can be something made from nothing by energy. Works for me anyhow, then again I don’t like infinities or Deities. But I’m not a physicist, Enjoy.
I have been led to this page trying also to feed my curiosity regarding certain questions relating to the universe. The questions and answers here are scholarly and professional, thoroughly learnt. Yet still the more I read i fear the answers we seek will never come from science or a scientist. To prove the existence of the theory you have to test the theory and create its formula to validate the theory. (Yes they are trying with the Hadron Collider) but if you only have the remains of the matter created by the original energy you are not working with all the data, and you probably will never have all the data until you figure out what started it all in the first place. Scientists are watching a film for a fragment of random time and have not even been told what film they are watching and they keep changing their minds consistently.
Did we not recognise that if matter and energy are one, and that all things are created from energy?
What in earth/universe could create the energy/mass needed to that would have resulted in the creation the universe?
Or ask yourself another question.
Why do we say that are universe was created?
If all things are energy and energy is matter does this not mean that the universe now occupies the same matter that existed before it but in different form? Would it not be wise for science to think outside the box (or pi circle). If they did they may figure out that the universe is not expanding unless the matter already exists, and remember folks…matter can’t live without energy and energy can’t live without matter.
What could make energy condense? Some sort of gravity? Too much energy packed in a closed space?
Gravity is not force . It is the mass which bends space and all other small mass gets attracted towards it . Same rule gets applied for all planets .
This is all quite a lot of fun to consider. We must always admit our severe limits of what we call “knowledge” and even abilities to “know”, but still keep considering things in different lights or perspectives. At the end of the day is night and another day to follow (hopefully).
It seems that we often look for models which in some way relate to our physical experience, hence more recent “string” theory, etc. Theoretical Physics invites us to think about our thinking. We may accept the invitation, and then …
I got poo on the wall. It condensed to a slow vibration from the energy radiating out of my butt. What a surprise…it’s on the wall because I couldn’t handle it.
Now I moved from Asia ( India ) to Europe and I have accomplished what I want in life. 👍👍👍👍👍
Now, I don’t care about this. I don’t care which planet is moving around which 😂 and all other nonsense stuff. After coming to Europe I realize that if your country has less population, pollution and corruption then you can enjoy life and that is the most important thing on the planet😂
To live a good life one required
1) Health ( physical / Mental )
2) 100 years of life
3) Independent life and
4) Money.
I wish in the future population of the Earth should become 12-15 CR and whoever will live at that time should live relax and enjoy life using Artificial Intelligence and without religion/cast and creeds belifs.
This concept is interesting except for the fact that we only distill information from what we are taught. If we begin to actually understand the basics then what we do know is that all particles (condensed energy) are fundamental components. Which do not change their energy (sometimes referred to as rest energy) I.e. that is the same energy as when the specific particle was created. That means particles cannot radiate energy away or even communicate data to any other energy system or they wouldn’t be fundamental. Being condensed energy, a particle can only exist in a part of space and no other particle can inhabit that space at the same time. So one has to ask the question where does the inertial energy due to kinetic thrust actually come from. You need two particles to create a kinetic thrust as well as conservation of energy and geometric laws. The conservation is an observed phenomenon and is also found mathematically to be a fundamental law. This law is actually what keeps our universe physically stable. The fact you need a minimum of two particles or at least a single particle and an alternative kinetic energy source to promote this conservation is why the universe is symmetrically balanced.
If you look carefully at all atomic structures and molecular material structures you will find that every part of the system comprises some form of charge, either in static form, or moving charges which each incorporate an integral and orthogonal magnetic field and in that form is called electromagnetic radiation. These systems are what totally comprise all particles of condensed energy thus all that we currently know can be thought of as various forms of charge. This will even apply to gravity which we believe is not allied to charge however it will be found very soon that gravity is actually a function of charge but doesn’t work the way we have envisioned it either from Newton to Einstein. As Physicists we need to learn to ask more questions and not just accept the answers as known. As an aside if you take the dimension of time you will find that it totally becomes a function of radiated local energy (and where does it radiate from?). This also presumes that time as a function is related to cosmic charge, Einstein understood this, as it was part of general relativity and then special relativity, but the characteristic could have been extrapolated by a better understanding of why this is the case as well as potential energy and kinetic energy have exactly the same dimensions except one is spacially static and the other spacially moving. My book on these topics will come out in another eight months.