The original question was: According to Gertrude Stein “There is no there there.” But is there “something” there? I guess we know that atoms are “there,” but what about the constituent parts? Electrons, protons, quarks, etc. Are they really “there,” or do we fall back on Heisenberg and say there is nothing really there, just a point particle at some probability? When and/or where is there finally something with a spatial dimension? At the Planck length? But my real question is this: If we could completely stop an elementary particle’s forward motion and say “there it is,” and then take a really, really high res picture of it (indulge me here), what would we see and what would it look like? A cloud of energy whizzing around looking like heat waves in the desert? A sphere made of “quark matter”? Or suppose we had a few billion-billion-billion pure quark particles stuck together in a jar (further indulge me here), what would we see?
Physicist: The best way to think about matter is as a “quantized field”, like an electric field (the “quanta” of the electric field are photons). Unfortunately, nothing really has a size on the atomic level. If you see an ocean wave you can ask “where is that wave?” or “how big is that wave?” and get a good ballpark answer. But when you start asking “where exactly is that wave?” you start running into problems (try it).

You can see more or less where the wave is, but can you say for sure where it is to within a millimeter? Hells no! Fundamental uncertainty!
The Heisenberg uncertainty principle isn’t so much about having uncertainty about some definite particle, it’s a statement about the nature of particles in general. That is to say, the particle itself is uncertain, not just the measurement apparatus. It’s not just that we can’t tell where a particle is, and how fast it’s moving, it’s that the particle isn’t actually at a particular place/speed. There’s a pretty nasty post here that covers how we can tell the difference.
Fortunately, it barely matters. You can never say exactly where a wave is, but that doesn’t change the fact that it’s there. You can still interact with it, and if you’re working on a large enough scale, the wave seems to be in a very definite (but not perfectly definite) place, and may as well be a particle.
A really high res picture would look like a blurry dot (sorry, no way around it).
Color, on the other hand, you can talk about. Matter can absorb, transmit, reflect, or emit light. For example: rocks absorb most light and reflect rock-colored light, glass transmits visible light and absorbs infrared and ultraviolet light, and glow in the dark stuff emits light.
In order to absorb light the material in question has to have energy levels such that the difference between two of them is equal to the energy of the incoming photon. For individual atoms this is just the atomic spectra. For more complex materials you get energy levels from the vibrations, and interactions between atoms. For something like a rock this covers pretty much every possible incoming energy, so it can absorb almost any kind of light.
There’s a lot of nasty mathematical physics that governs how likely a photon with a given energy is to be absorb, reflected, or transmitted. The situation is so complicated and difficult to predict, that (by far) the best way to figure out the optical properties of a material are to make that material and then look at it.
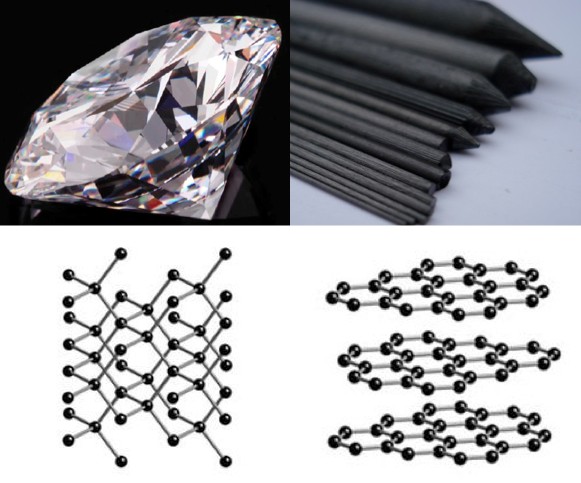
Diamond and graphite are made of exactly the same stuff (pure carbon). Their wildly different optical properties are caused entirely by how the atoms are arranged. If we hadn't dug this stuff out of the ground, I don't think anyone would have predicted this. Theoretical optical physics is nasty.
Due to quantum chromodynamics (one of the best buzzwords ever), a bunch of quarks will always appear in the form of larger composite particles (protons, neutrons, kaons, pions, etc). So being unable, even in theory, to make this stuff, you’re left with guesswork. With a wild guess, for not particularly good reasons, I would guess that a jar full of quarks would be clear in the visible spectrum.







In order to observe, energy in some form needs to be imparted on the object in question. For instance, to measure the resistance of a material, an Ohmmeter is used which flows a very small current through the resistor and then measures voltage across it. A small current (nA) is used so as not to adversely influence the material’s resistance. Heating it up with too high a measuring current may raise its resistance and lead to a wrongly measured value. Knowing volts and amps, we apply Ohm’s Law (V=I*R or R=V/I) and we get resistance which is displayed ion the Ohmmeter. Similarly, a stream of electrons, waves of light or X-rays, gamma waves, etc. are directed on relatively large objects (microscopic to our eye but larger than several layers of molecules). Such energy may not impact the object enough to disturb it but at much smaller scales (down at the molecular or even atomic level), we begin to affect the behaviour of what we’re observing. A stream of electrons or electromagnetic energy (E=h*f) begins to have meaningful impact on atoms. Atoms move and become agitated as we bombard them with the energy we need to direct at them in order to observe them. No energy – no observation. It becomes much more of a challenge and difficult to observe them. We need to rethink how to use the energy to make observations without disturbing them greatly and design more clever experiments and methods of measuring and observing. It can be challenging enough to do this at higher scales let alone smaller ones. Research at CERN’s Large Hadron Collider helps to answer these questions. Evidently, it’s mostly space (vacuum, nothing) between the nucleus and electron cloud of atoms.
So if things make no sense to us at the subatomic level, why not study the macro level in depth? Like electromagnetism explains reality a lot better than the nuclear force does. I understand that the nuclear force is stronger than electromagnetism and I believe the difference in strength is 137 fold, but that’s only at subatomic level. In other sense electromagnetism explains celestial bodies a lot better and reality in a macro scale.