The original question was: Is it possible to actually create a substance such as Kurt Vonnegut’s “Ice-9 (Nine)“, which could, in theory, bond with water (especially seawater) and replicate like a virus, freezing the oceans (or all liquid water on Earth) solid?
Physicist: Nope. There actually is an ice-IX, but there’s nothing that special about it.
Crystals in general are self-propagating. That is, once a crystal is present the raw materials in the environment find that falling into place in the crystal’s lattice results in a net drop in energy, but weirdly enough it usually takes a little energy to start a crystal from scratch. You can see this in action with super-cooled water: the water is cold enough that it should freeze, but no collection of water molecules is able to start the ice crystal.
The first tiny crystal that gets the ball rolling is called a “seed crystal”. Often it’s unnecessary because when a bunch of atoms are ready to crystallize small flaws in the environment can “look” like pieces of crystal to them, and they’ll just grab on to them (these are called “nucleation sites“).
It may seem strange to talk about a different kind of water-ice other than just regular “ice”, but keep in mind that many substances are perfectly happy to crystallize in multiple ways. For example, sapphires and rubies are made of exactly the same stuff, aluminum oxide (“aluminium” for our not-American readers), just put together in different ways due to the different environments that they form in.
Water in particular crystallizes in a surprising number of ways. To date, 15 different forms of water ice have been found, however here on Earth only one kind (“ice-I”) can form naturally. Even at the bottom of the Mariana Trench the pressure isn’t nearly high enough for any other kind of ice.
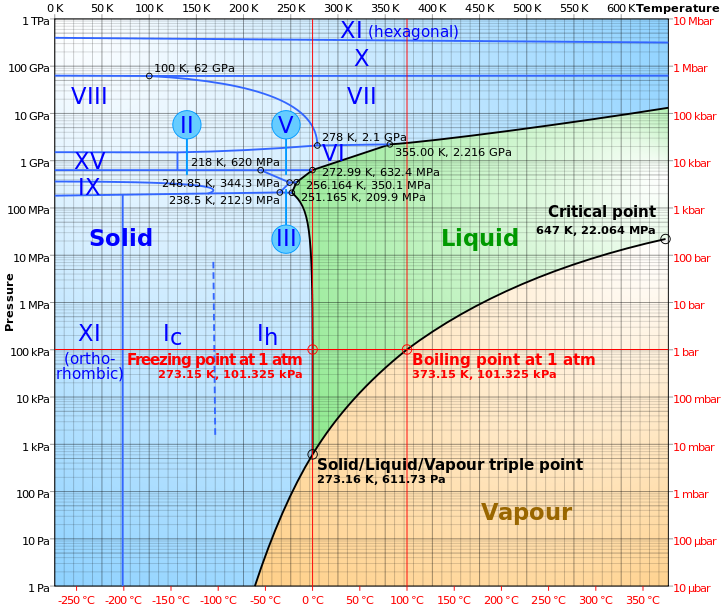
The different forms of water and ice at different pressures and temperatures. Sea-level air pressure is the horizontal red line.
A general rule of thumb is, if something wants to crystallize, and it’s in an environment where it can do so, eventually it does. Even if there’s no seed crystal already present, at some point a nucleation point is bound to show up (given enough time) and a crystal will form and grow.
The whole idea of Vonnegut’s ice-9 is that it’s a crystalline form of water, that grows rapidly at reasonable, Earth-like pressures and temperatures. If it could form at all, then at some time in the last several billion years, at some place in our gargantuan oceans, a tiny ice-9 crystal should have formed accidentally and encompassed the world.
We’ve already seen this on other, aptly named, ice-worlds. For example, Europa has lots of water, and is in an environment (pressure and temperature) conducive to the formation of ordinary ice-I. Not surprisingly, Europa long ago underwent a colossal “ice-9 type catastrophe”, in that all of its water (at least on the surface) quietly froze solid.
Long story short, if the Earth’s water could somehow be induced to crystallize at room-temperature, it almost certainly would have done so at its earliest convenience, a very long time ago.
The very cool super-cooled water gif is from here.









Incidentally, the inspiration for Cat’s Cradle came from cloud seeding, a technique pioneered by Vincent Schaefer, Irving Langmuir, and Bernard Vonnegut (a noted atmospheric scientist and brother of the aforementioned Kurt). Seed crystals (in the form of silver iodide) are released into the updraft of a thunderstorm by specially-equipped aircraft. These kick-start the formation of ice crystals in the cloud, leading to increased rain (because of the larger number of ice crystals to glom on to) and decreasing the amount of hail (because of the increased number of seeds, the competition for water molecules increases, and each individual seed is smaller on average, tending to become a rain drop instead of a hail stone).
Pingback: My Favorite Website! « Paya Farahmand
It is lucky the solid Ice I is lighter than the liquid otherwise a good portion of H2O would be locked up at the bottom of the oceans permanently.
If, like AC over DC, Nikola Tesla’s fluorescent light technology had likewise then prevailed over J.P. Morgan’s General Electric incandescent –heaters that just so happen to make light— how many mega-tons of carbon dioxide would be absent from today’s atmosphere?
Also, thinking of concrete locking up water like Ice Nine in Kurt Vonnegut’s “Cat’s Cradle”: How many tons of water are locked forever –barring asteroid-like Earth impacts and surface nuclear blasts– in the water-latticed molecules of concrete in the making of Hoover Dam? in whole federal interstate hwy system’s concrete? In the world, and how is that accelerating?
How many lbs. of carbon dioxide released in chemical reduction process of making cement for concrete?
http://open.salon.com/blog/anti-terrorust/2010/06/15/the_myopic_culture_of_the_automobile_concrete_and_oil
Pingback: The Freaky Physics of Supercooled Water - D-brief | DiscoverMagazine.com
Pingback: The Freaky Physics of Supercooled Water | Nagg
I don’t buy this explanation.
Since when does nature always “eventually accomplish” something that humans could?
Our planet had already been fully covered by ice during Marinoan glaciation.
There only meaningful difference between normal ice and Ice-9 of Vonnegut is in the temperature of crystallization. But Earth did have periods of the whole planet under zero in the past, so it was all covered in ice. But at some point after this the temperature on Earth rose and the ice melted.
If substance like Ice-9 could exist and Eareth would be fully covered on it, still there would be a possibility that the temperature will rise high enough to melt Ice-9.
Pingback: In Tiny Spaces, Boiling Water Stays Solid - D-brief
Pingback: Scientists Make Weird Type of Ice Halfway Between Solid and Liquid – Smithsonian | | All Breaking News
Leo is completely WRONG in saying that the only meaningful difference between ice I and Vonnegut’s Ice 9 is the crystallization temperature. If you read the book you would know that the crystalline structure is completely different and not natural, which is why the ‘seed’ crystals had to be man made. It is this difference in crystalline structure that causes the water to ‘freeze’ at lower temperatures.
Vonnegut’s Cat’s cradle was a warning. It is quite possible that modern man could or already has invented a crystal similar to Ice 9. Hopefully, if this is true, that crystal is locked away nice and safe somewhere. Modern man has the power or rather the responsibility to discover. It is in our DNA to research and seek out every answer to every question. The human ability to discover increased with the advent of computers. Today we can explore other planets…we can also blow ourselves up in glorious fashion, or destroy our planet just by burning fossil fuels and poisoning our environment. All the more reason not to put money hungry gangsters in public office.
Chris9 your point regarding gangsters in public office is to an extent and imo unavoidable. The entire concept of capitalism, again imo, needs a rethink and a redo because it just doesn’t work well anymore. Please forgive the diversion from Ice 9 for a moment and consider that greed, corruption, selfishness, confirmation bias, and the general tendency for automaton-like thinking in high and low sectors of government are creating, have created a huge disparity as manifested by a kind of gentrification that is unusual: the result of an adherence to dogma rather than any real effort to break people free from poor quality of life experiences over the lifespan as a result of not being committed to tackling the issues that would result in culture changes from the bottom up to enable prosperity based on merit rather than handouts which accomplish no lasting effect. As for Vonnegut’s concept of ICE9, I find the idea interesting and not without some scientific value.
I suppose this argument also rules out an oil-9 catastrophe.
It is a book.