Physicist: In a word; nope.
The Heisenberg Uncertainty Principle is a statement about how “certain” some combinations of quantities can be. The most commonly referenced is the “position and velocity” version of the Uncertainty Principle, that says that the more exact the position of a thing (any thing) the less certain its velocity, and vice versa. It’s basically because of the Uncertainty Principle that you’ll hear about how quantum mechanics predicts that “particles have some small chance of jumping across the universe (position uncertainty)”, or “there’s some possibility that all the atoms in a book will suddenly start moving and it’ll jump off the shelf (velocity uncertainty)”.
And in fact, if you apply Schrödinger’s equation directly (which essentially describes how quantum wave functions change with time), it does seem as through there should be no problems with things suddenly jumping around. If you apply it directly you find that if you have a particle confined to a particular region, then any amount of time later there’s some chance (no big) that it can be anywhere else, which is pretty exciting. Unfortunately, Schrödinger’s equation is an approximation in very much the same way that Newton’s equations of motion are approximations of the (correct) relativistic equations of motion.
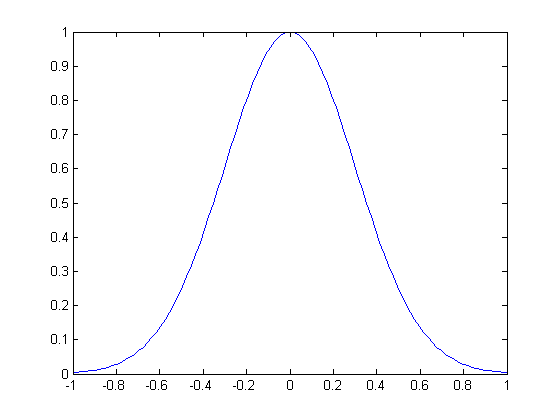
Soon after a particle’s position has been measured to be near zero, the wave function of a particle (which describes the probability of it being found at that position) tends to spread out like this, getting wider and wider as time goes on. According to Schrödinger’s equation the tails on both sides approach, but never quite reach, zero.
Schrödinger’s equation was a massive break through and provided a lot of insight into a lot of problems. But despite that, it doesn’t work perfectly. In general, if you have a theory and it doesn’t line up perfectly with special relativity, then you only have part of a theory. The fact that Schrödinger’s equation is “non-relativistic”, as evidenced by the fact that it predicts that sometimes particles will blink from place to place faster than light, made a lot of physicists extremely nervous. It took a couple more years (1926-1928) until Dirac fixed the problem with the Dirac equation, which is more or less the same, but adheres to relativity.
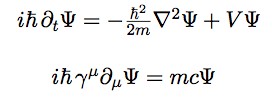
The Schrodinger Equation (top) and the Dirac equation (bottom). The Dirac equation takes into account relativity. Heck, it’s even got a “c” for light speed in there.
Newton’s equations of motion are very accurate, but only up until they disagree with relativity. For example, they imply that there’s nothing special about light speed, and you can totally go faster. Similarly, Schrödinger’s equation is remarkably accurate in most day-to-day, electron-shell type calculations, but makes big mistakes when relativity needs to be taken into account.
Long story short, even when considering the Uncertainty Principle, nothing can ever end up someplace else that would normally require faster than light travel.
As for books suddenly jumping off of shelves; the universe according to the laws of quantum mechanics is a seriously weird place. But ultimately, laws are laws. In this case, the conservation of momentum and energy.
If you take the predictions of quantum mechanics at face value (and why not?), everything that can happen does (in a very specific, many-worlds, sense). But that “can” is pretty iron-clad. Something that’s possible, even if it’s very unlikely, will happen in one some versions of the world*, but a book (or any other object) suddenly moving involves some extra energy suddenly being added to the universe, which is no good.
So, winning the lottery 75 times in a row, while making blind free throws for a couple weeks: sure. Books jumping off of shelves: ridiculous.
* “World” is definitely not the right word for this, because it evokes images of other dimensions à la Sliders and leads to general confusion. Neil Stephenson uses “narrative” which seems like as good a word as any, and hits a little closer to the mark.







Is it also not possible for an electron to jump from one place to another faster than light?
But a book could jump off of a shelf if enough atoms in the shelf were to all vibrate in the same direction at once, which is highly improbable (even ridiculous), but not prohibited by the laws of conservation of momentum and energy. I’d expect the shelf to get a little cooler and have a slight recoil, but no big, and it’s entirely classical.
@Nolan
Exactly right. It is not possible for an electron to jump from one place to another faster than light.
Well as usual it looks like fiction is being mixed with science in the attempt to explain how things work. As a long time student of physics i am wondering if i should re read the works of Conklin (science fiction writer) or Feynman (Nobel physicist)…one at a time or together.
After thought. My point is: that if you are dealing with “probabilities” of the numbers that are impossible to achieve, then why even mention them since they are beyond comprehension. In my opinion here we can gain a better sense of “reality” from philosophy and religion….quantum physics appears to be having a nervous break down.
I thought the question was referring to the way Quantum Electrodynamics is described where it assumes that at the ‘next instant in time’, the electron is allowed the possibility to be anywhere in the Universe?
Since it is not possible to determine the path of trajectory as well as the momentum of an electron as govern by Heisenberg Uncertainty principle….!!! Therefore, we use the concept of probability in order to determine its certainty…… Does the concept of probability breaks down ” Einstein Special Theory of Relativity “, by predicting that a particle can occupy two different position at the same time… which means that a particle has to travel(i.e. move back and fort) faster then the speed, for an observer to visualize that the same particle has occupied two different position at once !!!
At the scale that quantum mechanics is usually applied, The Physicist is totally right; everything that can happen does, and the universe is a pretty weird place. However, there is a “non-zero probability” that the exact right conditions will spontaneously occur to make a book jump off of the shelf. So in a way, yes, quantum mechanics says it is possible for some crazy stuff to happen (except, of course, for randomly jumping to a place before light). However, when we discuss the scale of books, the probability function would have to give the probability of every conceivable course of events. So, while the chance of the book flying off the shelf is “non-zero”, there are practically an infinite number of other non-zero probability outcomes that could happen. And, given that there must be a significant probability for the book to just sit there and do nothing (let’s assume it’s 99% in a hypothetically silly world), you can imagine that the probability for each crazy outcome would be (assuming each is equally likely) 1%/infinity (may actually be slightly smaller than infinity), because the 1% is divided among all the infinite random outcomes. So, it’s true, there’s a chance the book will fly off the shelf, but the chance of it doing specifically that is so small that I’d think it was crazier if I saw someone waiting for it to happen than if I saw it happen.
You say an electron can move faster than the speed of light. This only appears to be the case because all electrons are identical. Imagine a long tube filled with electrons. The tube stretches across across the universe. Now you squeeze in another electron at one end of the tube. This causes an electron to be squeezed out of the other end. The electron hasn’t travelled across the universe – it’s different but identical electron
@Alistair Nope, the main interaction of electrons with eachother happens through the electromagnetic field, whose gauge boson is the photon. The electron on the other side of the tube won’t pop out until light had the time to travel all the way through the tube.
So is there or isn’t there a possibility (no matter how infinitely small the probability is) of an object shifting locations? I didn’t quite get that from this post
Nope!
I, personally, go through periods were objects will shift in my presence… not that they weren’t already going to shift or didn’t have the ability to do so but.. I have noticed this and I have always experienced and witness small but notable weird things, the frequency and then when it stops. It’ll be something like a dish in the sink or a pencil will roll off of a table and I’m just in the room or near not engaging with the object or changing the airflow such that these small objects will shift. It starts slow, escalates, then dissipates and ceases for periods of time. What is the probability that one person would experience events such as this with a wave like pattern repeatedly? It makes me feel a little crazy that I even notice and even more so telling others about these incidents but I’ve always wondered why this happens.
What energy do you mean? Weak, strong, electric, gravitation, dark energy? I thought, the first two won’t matter, and the third is kinda cancelled out… and gravitation is not defined “quantum-like”, is it? So it’s basically only “dark energy” to save the day? But does dark energy work on this scale? And does quantum mechanics account for it?
why ?,if there is worm whole and all the sub atomic particle of the book pass through it down the floor
What about Quantum Entanglement/Quantum Teleportation? Doesn’t the fact that this is a proven fact and can at least be demonstrated in a lab by humans (though I don’t think we have observed or don’t have the ability yet to observe if this happens naturally, but we assume it does) mean that quantum particles can somehow travel faster than Einstein’s cosmic speed limit, apparently jumping to a new location with no regard to the speed of light?
I am by NO means a scientist, so if this is totally irrelevant to the idea here that all of the atoms in say, a book, could move or “jump” some distance by all moving in the same direction at the same time albeit with an infinitesimally small chance to ever do so, then forgive me. However in my thinking, it occurred to me that if Quantum Teleportation is a proven fact than doesn’t this have some bearing on whether or not this question’s answer is true or false, even if it could only occur once in a near infinite number of near infinite universes over a near infinite amount of time?
I say “near infinite” because if any of these were truly infinite, then like others mentioned, anything that can ever happen will happen at least once, and that means pretty much ANYTHING can and will happen at least once – from a room full of monkeys achieving an exact copy of the works of Shakespeare by randomly hitting keys on typewriters to a book jumping 3 nanometers or 3 cm to the right off of a shelf.
I realize the bizarre world of Quantum Mechanics does not always jive with the current models and theories of macro-physics as we observe and understand them at large in the universe, so is this a case of the two concepts (Quantum Teleportation and solid objects moving without any energy being applied simply from the smallest particles that make up the book all moving in the same direction at once) just not working together, or two completely irrelevant things? Thanks!
@Mike Plouffe
Entanglement and quantum teleportation are regular things in modern quantum labs (well-studied and pretty well understood), and are at least used in nature (how commonly it’s used in nature is still a very open question).
Unfortunately, they’re very different from the subject of this post. There’s a post here that describes how entanglement is used in quantum teleportation and why it doesn’t actually involve telepotation, or anything faster-than-light.
@The Physicist
Well I feel dumb, but hey, that’s how you learn, right? Thanks very much, I appreciate your comment and link to more info. 🙂
@ Nolan
Electrons as particles can’t travel faster than light but if information isn’t being gained on it then it isn’t a particle. Its a probability wave instead. Probability waves don’t have mass so aren’t restricted by the speed of light.
So you put an electron in a box, turn off your electron detector for a very short period of time, turn it back on and locate the electron. Repeat numerous times. In many instances you will find the electron somewhere in the box it couldn’t have reached yet unless it violated light speed. The reason is probabilities don’t have mass.
@BloodLust
It sounds like you’re talking about quantum tunneling. That does allow things to get to places that are “classically impossible”, but even then the speed of light is obeyed.
@ Cristy Howard
I’ve seen it too, more than once….long story. There is a spiritual realm & people pay upto 5k for Black Magic to be excercised on others. Have recently watched footage captured in one of our stores, it’s unbelieveable , one quite clearly see’s orbs of light darting around the storeroom, paperwork on the noticeboard lifting & an orb disappears into the wall & suddenly a bottle on a shelf against that wall comes flying off the shelf. Our State Manager was in awe asking how do we report this? Paranormal activity for stock loss isn’t on the form, they tried burning the footage to explain the stock loss but to no avail. My colleague played it on the CCTV & recorded it with her phone, she bought it in to show me. You’ve obviously Google objects spontaneously moving in hope science can explain but it can’t & they never will, it’s simply beyond comprehension
@ Valerie
Many, MANY people have reported witnessing objects suddenly moving – everything from a rocking chair start rocking on it’s own to relatively heavy objects being “thrown” across the room, for lack of a better term, such as a wine glass flying through the air and smashing into pieces against a wall 10 feet from where it was initially sitting on a counter. It’s fascinating that the camera picked up the floating orbs of light, which would seem to be some sort of energy somehow manifesting itself, occurring at the same time the manipulation of objects was happening, seeming to indicate that these two events were somehow connected. Some people will try to explain away events like these as malfunctioning cameras or reflections producing the orbs, and simple coincidence that objects moved at the same time, and that an earthquake tremor or something provides a rational answer for the objects behavior.
While sometimes this IS true, that there are completely normal and rational explanations for what at first appear to be bizarre and unexplainable events, people insisting that every incident like this can be explained away with known phenomena are simply arrogant and too closed minded to be working in any field of science.
The fact of the matter is that we, as humans, actually know very little about the universe and our own world compared to how much there is to know. I believe there is a scientific and rational explanation for everything, we just have yet to discover many of what they are yet. What is considered the realm of the paranormal is simply just what we don’t yet understand.
The internet and computer technology would have appeared to be magic to people a thousand years ago, but not because integrated circuitry and microchips are indeed magic, just because people back then lacked any knowledge or understanding of how these things work. We will eventually discover scientific explanations for everything, given enough time, and we may indeed find that the cause of what appears to be spiritual or “ghost” activity is indeed just that, but that explanation is considered rubbish today only because we lack the knowledge to understand it. Humans often think we’re the pinnacle of evolution in the universe and that we know everything today when we certainly don’t, and only by keeping an open mind can we discover the truth behind incidents like these – follow the evidence to the truth. Someday.
Only a fool thinks he knows everything – a truly wise man knows how little he knows.
If a book jumps off a shelf the far more likely explanation is that you live in California.
it’s hard to understand why a book couldn’t move if the observed stuff happened (quantum effect spotted in a visible object) in the following link:
http://physicsworld.com/cws/article/news/2010/mar/18/quantum-effect-spotted-in-a-visible-object
“As for books suddenly jumping off of shelves; the universe according to the laws of quantum mechanics is a seriously weird place. But ultimately, laws are laws. In this case, the conservation of momentum and energy. … but a book (or any other object) suddenly moving involves some extra energy suddenly being added to the universe, which is no good.”
The article assumes that the extra energy must be added to the universe for books to move. I disagree. Certainly the shelves could lose their energy, e.g. become very cold all the sudden, and that lost energy could have transferred to the books — thus, conserving the energy. The same thing with momentum: the shelves could cancel out the momentum of the jumping books.
Therefore, the jumping books should be allowed under Heisenberg’s Uncertainty Principle.
I understand the probability for a book jumping is almost 0.
But what is the probability for an electron to jump?
What for 2 e to jump?
What for a molecule? Or five molecules together?
A book cannot jump but could this phenomenon cause a blood vessel rupture throughout a lifetime?
Theoretical Physicist Michio Kaku disagrees here in this video. Says that math can calculate the exact possibility you will wake up on mars tomorrow.
http://bigthink.com/videos/parts-of-me-ooze-in-all-directions-2
Couldn’t quantum uncertainty make all the molecules making up a book suddenly disintegrate and spread in all sorts of directions at the speed of light, while exactly simultaneously quantum uncertainty makes an identical book emerge billions of light years away, seemingly from nowhere, as an inversion of said process, not caused by said event, but as pure coincidence, thus creating at least the “illusion” of a book “jumping” billions of light years in no time?
If so, we would only need to figure out how to cause those two things as a whole to happen with a ridiculuously much higher probability than other things that could happen, for it to be possible to actually make a book jump billions of light years in no time.
The reason why I think this might be possible is the fact that quantum entanglement can make one particle affect another particle’s spin in no time, even if the distance between the two particles is billions of light years. Why would it not be possible to learn how to create so many co-operating instances of such entanglement, and organized in such patterns, that the above “book jumping billions of light years in no time” takes place?
To make this easier, simplify the universe, Say it is 14 billion light years in diameter and only has the earth. Shoot an electron into space and wait about 30 billion years. Then this electron could literally be anywhere in the universe.
Then pull out your electron detector and find this electron . The moment you find the electron you have caused its wave function to collapse from the size of the whole universe to a little dot. So essentially the electron was everywhere existing in the whole universe and then it collapsed faster than the speed of light to a little dot on your detector. So next take a book and shoot it out into space at some huge velocity , short of the speed of light. If you wait long enough, you will have no idea where this book is. so it will essentially occupy the whole universe. When you detect the location of this book, billions of years later, you are collapsing its wave function just like the electron. So a book can move off the bookcase if you put it on the bookcase and don’t look at it and then come back trillions of years in the future to find it again. It’s location would be different.