Physicist: In very short: nope.
The second law of thermodynamics is sometimes (too succinctly) stated as “disorder increases over time”. That statement seems to hold true, what with mountains wearing down, machines breaking, and the inevitable, crushing march of time. But living things seem to be an exception. Plants can turn dirt (disordered) into more plants (order), and on a larger scale life has evolved from individual cells (fairly ordered) to big complicated critters (very ordered).
However, there are a couple things missing from the statement “disorder increases over time”, such as a solid definition of “disorder” (it’s entropy) and the often-dropped stipulation that the second law of thermodynamics only applies to closed systems.
Creatures, both in the context of growing and reproducing, and in the context of evolution are definitely not closed systems. Doing all of that certainly involves an increase in order, but at the expense of a much greater increase in disorder elsewhere. Specifically, we eat food which, with all of its carbohydrates and proteins, is fairly ordered, and produce lots of heat, sweat, and… whatnot. Food, and air, and whatnot are what make living things “open systems”.
If a creature could take, say, a kilogram of non-living, highly disordered material and turn it into a kilogram of highly ordered creature, then that would certainly be a big violation of the second law of thermodynamics. However, people (for example) consume along the lines of about 30 to 50 tons of food during the course of a lifetime. Some of that goes into building a fine and foxy body, but most of it goes into powering that body and fighting degradation (blood and skin and really everything wears out and needs to be replaced). So, about 0.15% (give or take) of that food matter is used to build a body, and 99.85% is used for power and to fight the entropy drop involved in body construction and temporarily holding back the horrifying ravages of time.
When compared to the entropy involved with turning food into the many, many bodies that make up a species, evolution is barely an afterthought. In fact, the entropy (as used/defined in thermodynamics) of most animals (by weight) is all about the same. A person and a mountain lion have about the same entropy as each other, simply because we weigh about the same.
The big exception is photosynthesizing plants. They really can turn a kilogram of inert, high-disorder dirt, air, and water into a kilogram of low-disorder plant matter. But, again, they’re working with a bigger system than just the “plant/dirt/air/water system”.
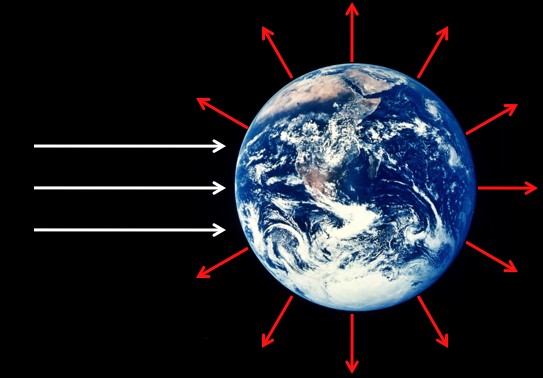
There’s a huge increase in entropy between the incoming sunlight and the outgoing heat that’s radiated away from the Earth.
Sunlight is a bunch of high-energy photons coming from one direction, which involves relatively little entropy. A little later that energy is re-radiated from the Earth as heat, which is the same amount energy spread over substantially more photons and involves a lot more entropy (relatively). This huge increase in entropy, between the incoming sunlight and the outgoing heat, is the “entropy sink” that makes all life on Earth possible (with just a handful of exceptions). In particular, green plants take a tiny amount of the sunlight that hits the Earth and turns some of the energy into sugars and other useful plant-ey material. It all eventually turns into heat and radiates away, but instead of doing it all at once it does it through a few links in the food chain.
You can think of this huge sunlight-to-re-radiated-heat increase in entropy like water going over a waterfall, and life as being like a hydro-electric dam. It all ends up at the bottom of the falls, but sometimes it can do some interesting stuff (life and other useful mechanical work) on the way.








@itsnobody
If religiosity promoted health then sub-Saharan Africa would be the healthiest place on Earth. Residents of highly secular Sweden (life expectancy of 81 years) and Japan (life expectancy of 82 years) outlive the more religious Americans (78.5 years). And, by the way, the life expectancy in China is 75.2 years now. The problem with your numbers and these numbers is that this has nothing to do with religiosity. You are committing the sin of bearing false witness and the logical fallacy of Post hoc ergo propter hoc (aka correlation is not causation).
itsnobody wrote: “I don’t see any explosions decreasing entropy, do you?”
As a matter of fact, yes I do. When Hydrogen oxidizes (explosively) there is a vast drop in the entropy of the atoms with the heat being released into the air molecules. So yes, I absolutely see “explosions decreasing entropy” locally. If you are as educated as you claim how can you possibly miss this?
And we can calculate the exact change in entropy in the micro-explosions such as the process of ATP -> ADP hydrolysis: http://chemwiki.ucdavis.edu/index.php?title=Biological_Chemistry/Biochemical_Energy/ATP%2F%2FADP
This is elementary science.
itsnobody wrote: “I don’t see any engineers in any engineering field going with the crackpot evolutionist idea of “it’s an open system the 2LOT doesn’t apply”.”
Never heard of an air conditioner? Or the Peltier effect? Or the entropy of chemical reactions? NOBODY says 2nd Law doesn’t apply – what they are telling you is that you have to apply it to the WHOLE system — not just one tiny piece of the system.
I think it is clear that you haven’t studied thermodynamics at all.
@Dark Star “The entropy, which is the level of disorder, of ADP is greater than that of ATP. Therefore, due to thermodynamics, the reaction spontaneously occurs because it wants to be at a higher entropy level.” Going from ATP to ADP is a spontaneous increase of entropy, which means that it’s a micro explosion increasing entropy. Hydrogen reacting with oxygen also is an increase in entropy for the products.
The 2LOT is much more complex than just saying “entropy increases”. The statement “entropy always increases” applies only to closed systems, and that is only a part of the 2LOT. Furthermore, entropy is not always accurately represented as disorder. In classical thermodynamics, which I studied, entropy change is defined as the change of total energy minus the change in energy that is available for work.
It doesn’t sound to me as if anyone posting here has studied Thermodynamics to a great extent. That is not a slam, because thermodynamics is an arduous study program. its nobody seems to have talked to engineers who have and his comments are basically correct. I recommend that you all read the comment at the end of the chapter on the Second Law of Thermodynamics in the textbook of Classical Thermodynamics by Gordon Van Wylen and Richard Sonntag. I am not at home so I can’t give you the quote or the exact book title from my copy at this time.
http://www.eoht.info/page/Gordon+Van+Wylen
That page I linked and then you quoted is from UC Davis that looks at the exact entropy change and yes, considering entropy as ‘disorder’ doesn’t tell the whole story but neither is it entirely inaccurate (nor does ‘unavailable for work’ tell the whole story, the MATH tells the story). And yes, we all know it applies only to a “closed system” which is exactly what the theists posting here are missing — that’s what we’re pointing out to them.
You missed where I stressed there is a LOCAL decrease in entropy — while at the same time the TOTAL entropy increases.
So nothing you said really addresses anything I stated.
I think that if you re-read it more carefully you will see that what I said and the linked information is both accurate and in agreement with thermodynamic principles.
Pingback: “Mammal-like Reptile” is simply wrong…Extinctions may be more rare than you think and marine “reptiles” may not be reptiles either… | Digging up the future...
Pingback: Candidates misunderstand laws of Thermodynamics « KaiserScience
@itsnobody
According to your own arguments, life shouldn’t be possible either since it would violate 2LOT. According to your arguments, 2LOT cannot be true. You also speak about plants having pre-set system. One that doesn’t exist, by the way.
Can someone give me a clear and accurate definition of entropy? I haven’t been able to find one yet, and it seems very important for understanding the laws of thermodynamics.
@Benson that isn’t an easy question to answer, because it is very context-sensitive, but I’ll give it a shot. I think it’s easiest to boil down the various way of thinking about entropy into two quick answers:
1) Entropy measures the “disorderliness” of an object or conglomeration of objects.
2) Entropy measures energy that is lost to a system and no is longer available to do work.
I think these two definitions cover most of the ways we non-engineers use the term. To expand on the definitions a little:
1) An example is an egg. There are countless ways the individual bits of an egg can be configured that, on a macroscopic view, we would call “ordered.” For example, you could swap two bits of shell with each other, or two bits of yolk, and it’s still an ordered egg. But there are many, many, many more ways to configure it that are “disordered.” Take that example to the extreme, and it might be easier to visualize–if you take the billions of bits of egg and configure them in random ways, you’ll see that there are relatively very few configurations that we would call an ordered egg. (For example, any configuration that doesn’t have shell as the outermost part of the egg is disordered.)
So as the universe goes on its merry way, with bits of it interacting with other bits, there are many more ways that the resulting interactions yields less order. That’s entropy, and a simple way of envisioning why it seems that entropy either stays the same or increases, but doesn’t decrease. (The definition and egg example (though I’ve changed it up) are from Sean Carroll’s book, From Eternity to Here.)
2) I’m not sure there’s much I can do to expand on this, but there are two important things to remember, in the context of the discussion of 2LOT and evolution:
a) 2LOT addresses the entropy of an entire system, and life on earth is part of a system which includes the sun (because this is the source of energy for plantlife, without with there would be no animal life). It isn’t accurate to say, “In a growing plant order is increasing (entropy is decreasing) so this is a violation of 2LOT–checkmate atheists!” This ignores that the sun is part of the system feeding the plants, and the energy that radiates away from earth (no longer able to do the “work” of growing plants) is wayyyyy more than the energy captured by the plants to do that growing. So 2LOT is not violated because the entropy of the *entire system* has increased, just like 2LOT says.
b)2LOT does NOT say that entropy will ALWAYS increase in EVERY part of a system; it only says that the OVERALL, or NET entropy will remain the same or increase. So you can have *pockets* of decreased entropy (a growing plant) in a *system* of increasing entropy (the plant-earth-sun system).
Darkstar: “Japan (life expectancy of 82 years) outlive the more religious Americans (78.5 years).” “If religiosity promoted health then sub-Saharan Africa would be the healthiest place on Earth. ”
The longest living people in the entire united states are the 7th Day Adventists. So some religions, DO, promote health. And I’m pretty sure people are sick and tired of unthinking ‘I hate all religion’ people out there.
Arguments of entropy are compelling…really made me think. In a cosmological sense the law of entropy is preserved. However, Life on earth seems to counter the entropy argument. Simple systems combining into complex forms. While mathematicians can make arguments how this in thermo dynamically not a big deal on the Macro level…I find it interesting on a molecular level especially the thermo dynamics of DNA and cellular physiology…thoughts?
@Jason
Indeed…and the arguments here made me think more.
The fact that in our so called “closed system” Earth the birth of life looks like a miracle and the somehow spontaneous increase of order is fascinating. It looks like it goes against physics law, the 2nd law of thermodynamics…
So the scientist than explain this by putting things in a larger context, a larger system and the law seems to apply again…
But hey…if you really want to put things in larger system, than what do we know about the energy that we release out in space? What happense at the edge of the universe and multi-verse?
What if the energy the we gove away from earth (with entropy) goes into a larger system outside the universe and creates more Life (lower entropy).
Where does the energy come that created the so called Bigbang?
What if the energy is infinite in the multi-verse?
Or what if the energy is somehow finite but goes into cycles of:
Disorder > Life > Disorder > Life > Disorder > Life
The thing is, we don’t know what we don’t know. And the so called “infinite” has a difficult way to get explained in physics laws where all tend to be seen as a “finite” system.
@23rds
There are no statistics to support your claims.
@Antonio de Marinis,
You are greatly misunderstanding the 2nd Law of Thermodynamics. The situation is not that scientists rethink the problem in terms of a bigger system and then reapply the law. The situation is that the 2nd Law of Thermodynamics already states in itself that the overall entropy of the UNIVERSE increases because by definition, the universe is the only closed system that exists. You mention the multiverse, but the problems with that are:
1) It is not confirmed that a multiverse exists. All scientific theories rely on the one universe assumption because most of the evidence leads to that assumption.
2) It has been stated by scientists that if a multiverse does indeed exist, then the laws of these universes are unintelligible and radically different, to the point where the 2nd Law of Thermodynamics would not apply outside our universe anyways. So the existence of a multiverse is completely irrelevant.
There are already ways to explain where does the energy at the Big Bang come from. The main idea is to understand that the principle of causality does not apply at the singularity because time space curves do not exist yet: they are created after the Big Bang occurs. Once you get rid of causality, energy does not need to come from anywhere at all. In fact, it cannot come from anywhere. It just has to exist.
It is not true that infinities get a hard way of explanation in physics. So many infinite quantities are easily explained. The range of the electromagnetic and gravitational forces is infinite. The curvature at the event of horizons of black holes are also infinite. Using calculus, you are able to deal with these infinite points.
I get tired of seeing people ask about the 2LOT regarding life/evolution and just being dismissed with “that’s only for closed systems.”
Life and evolution don’t just start with some magic soup spontaneously generating life one day on a rock orbiting a star. That’s cheating. Why? Because you’re inexplicably starting with organized systems and an oh-so-convenient outside energy source. In reality though, the star and the rock and the magic soup all allegedly evolved from simpler chemicals to get to that point, so what about them? How did they come to be, in violation of the 2LOT?
At the end of the day, somebody needs to address the fact that THE UNIVERSE IS A CLOSED SYSTEM. So how does an explosion of hot quarks come to order itself into giant fusion reactors and distinct globes of orbiting matter ready to produce life (with one globe having generated enough life that it has overrun the planet.)
THAT is what I want someone to answer.
Rob, it has been answered here and everywhere else this question comes up.
2LOT does not say that every single bit of the system will increase in entropy. The entropy of the universe **as a whole** has increased while **pockets** of it have seen decreased entropy. This is not a violation of 2LOT.
The question can be asked in very simple terms—and these answers aren’t satisfying that:
If entropy is a fundamental principle of our universe, how is it that our universe is filled with complex systems?
In other words, the form of the universe itself demands that there must be another principle that defies entropy. But no one seems to want to acknowledge that.
Flange,
There is nothing about entropy which says there cannot be be complex systems. It says the system *as a whole* will trend toward higher entropy, and it is doing just that. It doesn’t say every single bit of it must be increasing in entropy at all times, so no “defying” principle is necessary.
@Flange the Flee:
Perhaps you haven’t read the comments well enough. Entropy is fundamental principle in the universe, but it only applies to closed systems, and the only closed system that exists arguably is the universe itself, which means that complex systems operating in decreasing entropy are possible, given that these systems are subsets of universe, a case which is true for our discussion. There are multiple principles that defy entropy. The fundamental forces of nature and the quantum mechanics are examples of this.
@Rob:
The fundamental forces of the universe and quantum theory explain how complex systems came to be within a closed system.
I fail to understand why electro magnetism(light) does not lose energy as it osilates back and forth through the universe. This would explain why light eventually goes red after traveling billions of light years. Instead of calculating the expansion speed of the universe, why not use the change in color (frequency) to calculate the entropy or loss of energy in each individual photon? Perhaps that lost energy actually turns into dark matter. As an aside I also think black holes converts dark matter into electrons and positrons which then use the Hawkins Radiation to increase matter into the visible universe. What do you think?
The fact is that the scientist that upheld the theory of evolution also accept the philosophy of entropy which assert that matter moves in the direction of disorganization but not organization.Why?
Pingback: Termodinamika Qanunları və Təkamüllə əlaqəsi | Rezonans
human disobeying the law of thermodynamics?
I still think light loses energy over a billion light-years of travel and that is why it turns red making our scientific community think it’s the universe expanding.
Isn’t the simplest way to convey the idea of how complex living systems evolved, making order out of chaos, that of an energy trap? The heat energy of the sun being a garden hose spraying a rockery that catches water at different levels of potential energy above the ground can be analogised as exciting molecules that are “caught” at stable higher energy configurations (the negative delta G of chemistry). Then it’s down to probability, evolutionary theory and an enormous time scale to get to chlorophyll, glucose, etc, etc… Temporary pockets of negative entropy if you will.
good!
My question isn’t about evolution. It’s about the universe forming into systems. How does that work with the 2LOT? The intuition about an explosion (i.e., the Big Bang), is that it’s an entropy explosion – everything coming out of it should be chaos and disorder and only continue from there.
So how do galaxies, solar systems, planets and everything on them arise from this disorder? Hell, where does gravity and it’s coalescence in specific areas come from? How do these behaviors work with the 2LOT?
Disclaimer: I’m not a physicist.
For one thing, the BB isn’t really an explosion–it’s an expansion. And I’m not really sure what you mean by “entropy explosion” but I don’t think that is the best label, either.
But even allowing an entropy explosion, that doesn’t mean that “everything” coming out of it should be chaos and disorder. 2LOt only says that the entropy of the entire system will tend to increase (and notice that word “tend”). There can be pockets of decreased entropy while the system as a whole increases in entropy. We see that happen today in many systems.
An explosion *is* expansion. I would say the only difference between something we generally call expansion and something we generally call an explosion is the speed. 🙂
I was being glib with “entropy explosion,” phrase, but I’m assuming the problem here is that despite its ubiquity, the 2lot is fairly broadly applied (I actually just reached the section on entropy in ‘The Information: A History, A Theory, A Flood that discusses this).
But if we do look at the BB as an explosion (i.e., a fairly chaotic almost instantaneous scattering of material), one doesn’t expect the formulation of complex entities to arise from that, especially in a universe that tends towards greater entropy and a place where as energy is transferred or transformed, more and more of it is wasted or made unavailable to create more complex entities.
So what makes this random scattering of material magically form up into complex entities?
Remember, the question is “why do living things not violate 2LOT?” It isn’t “why should we *expect* complex things to form?” We don’t really have to “expect” complex entities arising from something like the BB, but should we think they’re not possible because they violate something like 2LOT? No, and the simplest answer is because 2LOT does not say entropy MUST increase, or that it will increase in EVERY part of a system. Living things are examples of complexity arising in isolated areas in a larger system that as a whole increases in entropy. That does not violate 2LOT.
Haha, “remember, the question is…”
Literally my first sentence of my first post told you *my* question wasn’t about evolution. If anything I’m hijacking the comment thread. 🙂
So ok, I accept that the 2lot doesn’t disallow the rise of complex entities. I see you (Non Credenti), have brought your same response to at least two other people who have raised the same question: “the 2lot doesn’t say entropy MUST increase.”
Great, no problem there. Now we understand the 2lot. But the much more interesting question remains, what is working *against* entropy? If the tendency of the closed-universe-system is towards heat death (and it’s a pretty strong tendency to say the least), what’s the law of emergent complexity?
Christopher: models of the Big Bang indicate extraordinarily low entropy at the beginnings.
Someone with a better background in physics would give a better answer–and possibly tell me I’m off base, anyway, so take this with a huge grain of salt–but in short I’d say: gravity, the strong and weak nuclear forces, the electromagnetic force.
Whenever it seems that entropy is reversing, there’s always some system you’re missing. For example, when a big cloud of gas collapses to form a star, there’s a big drop in entropy. But at the same time, that mass heats up and ultimately sprays photons into space (both of which involve big increases in entropy).
A particle’s (or system’s) potential energy involves some low entropy, because when that energy is released there’s a corresponding increase in entropy. For example, it takes four hydrogen atoms to fuse together to form one helium atom, so you’d expect there to be a four-fold decrease in entropy. But that fusion also involves the production of lots of new particles; a couple neutrinos and typically a hell of a lot of photons.
So sure, the early universe was pretty chaotic. But it’s a lot worse now.
I read this article while releasing whatnot. I just increased entropy!
I have read through all of this interesting and intelligent conversation above. Its one of those things you find yourself doing on a cold rainy afternoon in the midst of a viral pandemic with lots of time on your hands.
The 2LoT has been called a “Law” since it was announced as a “Law” over 170 years ago. In reality it cannot fit the definition of a “law” but is more of a “principle” of thermodynamics. When any “law” has so many special cases and exceptions needed to explain away the things that appear to be, it must be demoted from it’s exalted throne as a “LAW” of science to the level of a “principle”. By definition a ‘principle” describes a specific phenomenon that requires clarity and explanation. Certainly this is the case in spades based on the above great discussion.
The problems and special cases with the “2LoT” are many. 1. Over the last 13+ billion years we have progressed from a plasma of subatomic particles into an organized Periodic Table of the Elements, the current building blocks of all matter, with far far greater order and complexity at all levels. 2. As a side effect of that above increased order occurring, many black holes and worm hole links through space have likely been created increasing the complexity and structure of space time along with massive gravitational energy fields created, exerting such power that even photons cannot escape. 3. Ditto for “dark matter” and it’s probable link in creating the web-like, actually very neuron-like structure of the lit-up universe with galaxies, clusters, super-clusters, mega-clusters on and on in scale as far out into the universe as we can probe – again increasing complexity and apparent order and structure within the entire “closed” universe as we can measure it. 4. And , especially in what appears to be the bio-centric tendency of our universe and life . A universe that is built from the quantum level up in complexity and strangely seems to be dependent on having a biological observer (a consciousness), a greater order present to even exist.
I mean, we have come a long way in understanding the complexities and very strange ordering of our reality from a couple of guys 170+ years ago noticing that a glass of hot water cools down by itself and coming up with a “Law” for “closed systems”. BTW, I don’t believe that there are “closed systems”. It’s a Newtonian illusion. Everything is always entangled and linked to everything else.
Just sayin’, its time to stop defending the 2LoT as a “law” with so many special cases and exceptions. A principle, yes.
I too have been doing research during these dark days of COVID-19. I am delighted to find this forum, as I have thought about these questions for most of my adult life. I encourage everyone to review the Wikipedia page entitled “Entropy and Life”. The apparent dichotomy between the development of living things and 2LOT is referred to as Schrodinger’s Paradox (1944 What is Life?).
Clearly there is general agreement that in fact the increasing complexity and organization of living things does not constitute a violation of 2LOT. For one thing, the environment here on Earth is not a “closed system,” and for another, the law does not preclude “pockets” of decreasing entropy within an overall high-entropy system.
But this does not address the questions raised by Flange the Flea (11/22/2016) and Christopher (5/10/2019), who ask for confirmation of some sort of anti-entropy force. The challenge here is that this is no longer a scientific question. We can empirically see the evidence of evolution in increasing complexity, organization and intelligence, but science cannot tell why this should be true. So in fact we must move beyond science and begin to consider religious questions. My synthesis is “In the battle against entropy, God and Evolution are on the same side.”
In his book “The Phenomenon of Man”, Pierre Teilhard de Chardin proposed three transitional threshold events in the history of life on Earth. The first was the inception of life itself (see “abiogenesis”), the second was the development of consciousness, and the third, still in the future, will be the Omega Point when all humankind unifies into a single entity. Using the term “noosphere”, he anticipated todays Internet and social media, which are starting to form a “Global Brain”. I can only hope that someday we humans wise up and start using Gaia’s abundant resources in a sustainable manner.
I don’t understand how chemicals can get together in just the right amount along with some electricity to create the first living cell. And from there, cells becoming more organized & not think this disobeys the laws of thermodynamics!
Hello Walter,
As stated above, there are no 2LOT violations here (Earth is not “closed”, there can be “pockets”, and then there’s the whole logical tangle of reversible versus irreversible interactions).
But to answer your question, it’s all about vast periods of TIME. Over billions of years, intelligent crystals, mega-molecules and organic proteins will form, to the extent that the underlying raw materials and energy sources are provided. Just like the monkeys who type Shakespeare’s plays. Here we are!
The metaphor of high entropy as chaos or disorder and low entropy as order or structure can be super misleading to laypeople. We describe the early universe as chaotic, and there was no structure to speak of, but entropy was extremely low then. Low entropy is not intrinsically conducive to life, and it’s not equivalent to complex structures. Stars are very simple structures, and there’s no life (that we know of) on the sun, even though entropy there is vastly lower than here on Earth. The observable universe didn’t emerge maximally complex and continually lose complexity. Complexity is a property that in some cases we could compare with entropy, but that analogy shouldn’t be pushed beyond its useful contexts.
Here’s a heuristic that most of us could probably follow: when people (who have studied a subject for far longer and know far more about it than you) seem to hold an absurd-seeming consensus, it’s probably wiser to try to figure out what they know that you don’t know than to instantly conclude they must be incompetent or conspiring to hide the truth.
Excellent points. Your heuristic is especially appropriate.