Physicist: If you wait forever, then you might see something happen. But the more practical answer is: no.
The universe does a lot of stuff (for example, whatever you did today), but literally everything that ever happens increases entropy. In some sense, the increase of entropy is equivalent to the statement “whatever the most overwhelmingly likely thing is, that’s the thing that will happen”. For example, if you pop a balloon there’s a chance that all of the air inside of it will stay where it is, but it is overwhelmingly more likely that it will spread out and mix with the other air in the room. Similarly (but a little harder to picture), energy also spreads out. In particular, heat energy always flows from the hotter to the cooler until everything is at the same temperature (hence the name: “thermodynamics”).
If you get in front of that flow you can get some work done.
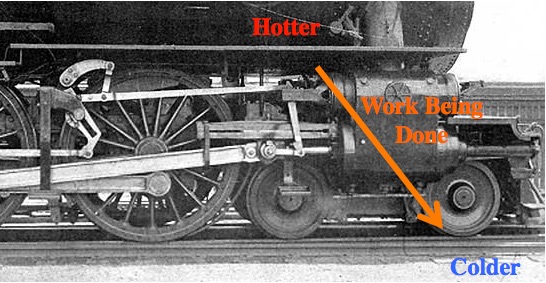
All machines need to be between a “source” and a “sink”. If the source and sink are at the same temperature, then there’s no reason for energy to flow and the machine won’t work. For example, if the water were already steam (not previously cold), then it won’t expand and you can’t use it to do work.
“Usable energy” is energy that hasn’t spread out yet. For example, the Sun has lots of heat energy in one (relativity small) place. Ironically, if you were in the middle of the Sun, that energy wouldn’t be accessible because there’s nowhere colder for it to flow (nearby).
The spreading out of energy can be described using entropy. When energy is completely and evenly spread out and the temperatures are the same everywhere, then the system is in a “maximal entropy state” and there is no remaining useable energy. This situation is a little like building a water wheel in the middle of the ocean: there’s plenty of water (energy), but it’s not “falling” from a higher level to a lower level so we can’t use it.

Useable energy requires an imbalance. If all the water were at the same level there would be no way to use it for power.
The increase of entropy is a “statistical law” rather than a physical law. You’ll never see an electron suddenly vanish and you’ll never see something moving faster than light because those events would violate a physical law. On the other hand, you’ll never see a broken glass suddenly reassemble, not because it’s impossible, but because it’s super unlikely. A spontaneously unbreaking glass isn’t physically impossible, it’s statistically impossible.
However, when you look at really, really small systems you find that entropy will sometimes decrease. This is made more explicit in the “fluctuation theorem“, which says that the probability of a system suddenly having a drop in entropy decreases exponentially with size of the drop.
For example, if you take a fistful of coins that were in a random arrangement of heads and tails and toss them on a table, there’s a chance that they’ll all land on heads. That’s a decrease in the entropy of their faces, and there is absolutely no reason for that not to happen, other than being unlikely. But if you do the same thing with two fistfuls of coins it’s not twice as unlikely, it’s “squared as unlikely” (that should be a phrase). 10 coins all landing on heads has a probability of about 1/1,000, and the probability of 20 coins all landing on heads is about 1/1,000,000 = (1/1,000)2. The fluctuation theorem is a lot more subtle, but that’s the basic sorta-idea.
The “heat death of the universe” is what you get when you starting talking about the repercussions from ever-increasing entropy and never stop asking “and then what?”. Eventually every form of useable energy gets exhausted; every kind of energy ends up more-or-less evenly distributed and without an imbalance there’s no reason for it to flow anywhere or do work. “Heat death” doesn’t necessarily mean that there’s no heat, just no concentrations of heat.
But even in this nightmare of homogeneity we can expect occasional, local decreases in entropy. Just as there’s a chance that a broken glass will unbreak, there’s a chance that a pile of ash will unburn, and there’s a chance that a young (fully-fueled) star will accidentally form from an fantastically unlikely collection of scraps. There’s even a chance of a fulling functioning brain spontaneously forming. But just to be clear, these are all really unlikely. Really, really unlikely. As in “in an infinite universe over an infinite amount of time… maybe“. We do see entropy reverse, but only in tiny quantities (like fistfuls of coins or the arrangements of a few individual molecules). Something like the air on one side of a room (that’s in thermal equilibrium) suddenly getting 1° warmer while the other gets 1° colder would literally be the least likely thing that’s ever happened. The universe suddenly “rebooting” after the heat death is… less likely than that. Multivac interventions notwithstanding.
Those events that look like decreases in entropy have always been demonstrated to be either a matter of not taking everything into account or just being wrong.

Fun fact: Patents for perpetual motion machines are the only patents that require a working model. Yet another example of the scientific conspiracy at work!
Long story short: yes, after the heat death there should still be occasional spontaneous reversals of entropy, but they’ll happen exactly as often as you might expect. If you break a glass, don’t hold your breath. Get a new glass.







Well, one of my pet ideas is that you could send Oases into the distant future based on relativity.
Another thought is that by responding to spikes in the behaviour of very small quantities of gas, you could effectively have a sort of cell-based thermodynamic resistor of sorts, (one idea I have is that this says something about the relation between a gas and a solid sort of).
Just thought I’d share:)
Just to clarify, when you say “‘Heat death’ doesn’t necessarily mean that there’s no heat, just no concentrations of heat,” are you talking about heat–the transfer of thermal energy–or about thermal energy itself?
I understand, perhaps incorrectly, that life for example is a low entropy state the formation of which requires energy which increase the universes overall entropy?
To quote Dumb and Dumber, “so you’re telling me there’s a chance.” I was wondering, does the notion of time depend on entropy? If there’s no more source or sink for energy, then does time still even exist? If the energy can fluctuate randomly though, can’t disappear, and enough time passes, then could not the remotest of all possibilities still become more than likely to happen? Sort of like how there’s more than a 50% chance that two people in a room of 30 will have the same birthday. Or like Douglas Adams improbability engine. I’m thinking along the lines though of all the energy gathering into a single point of order to create a source like the Big Bang 2.0. Could that be possible, perhaps after all other possibilities exhausted themselves? Or if you redefine the length of time it took for that to happen as a single smallest unit of time on some grander scale of events, then what would time passing seem like?
Just to add one more thought, it might not be so much that all the order of the universe in the Big Bang would spontaneously emerge of its own accord, if it could be more likely that some simpler structured triggers spontaneously emerge and lead to larger chains of events that cause the order of the Big Bang to happen. I guess the thought is, instead of a brain spontaneously emerging fully formed, that all of the structure inherent in the evolution and development of something simpler such as a cellular organism and it’s environmental pressures might be statistically more likely to spontaneously emerge. Then that could lead to the brain forming. But instead of the cell emerging spontaneously, maybe it’s more statistically likely for just atoms and environmental pressures akin to the laws of physics to emerge, that could lead to the cell. And so on back to suppose, if doing so might lessen the burden of the part that should have to emerge spontaneously.
i think the first sentence seems wrong. 1) if the U approaches thermal equilibrium asymptotically, you can always see something happen by waiting long enough. 2) (as the article itself describes) there are fluctuations that can locally increase entropy, and that would seem like something happening.
Hey, but isn’t at this precise moment the Universe expanding and then it will be shrinking and when it finally shrinks there will be another Big Bang so the universe dies and borns eternally?
So… expanding universe = eventual heat death via entropy. Energy levels everywhere are equal… But what drives that expansion? If it were only the laws of motion the centre of the universe could be identified – the origin point. But as everything is moving away from everything else, that implies that other forces are at work as well. Pressures from stars and the like. But once those are gone, I suspect that the one active force left in the universe will start having a greater effect.
Gravity. While negligible in the current greater universe, its very nature is contrary to expansion. Eventually, over a similar timeframe as the life of the universe, super gravitational forces will have a greater effect, first on their surroundings, then on each other. This will continue until all that exists once again is at a single point, recreating the conditions of our Big Bang.
Or I could have no idea of what I’m talking about and am just throwing out an idea.
“However, when you look at really, really small systems you find that entropy will sometimes increase.” Shouldn’t be, “decrease”?
The answer given must be predicated on the assumption that the universe is not infinite. Otherwise, ‘very very unlikely’ x infinite time x infinite space = inevitable (eventually, somewhere), surely?
In an infinite universe over infinite time, all improbable events are inevitable. Loose atoms will randomly collide to form anything and everything, including big bangs and sentient creatures to make sense of it all. And a flock of pink geese riding a unicycle, naturally.
I have a theory that we are already at the end stage of the universe. Currently the only sources of heat and energy are stars while rest of the Universe is cold and dead, but it may not have been like this in the past.
My theory is that once all the energy is turned into heat, gravity comes into play, and all the heat is brought back to the center and get more and more intense. Eventually, there is too much and it results in another big bang. matter is spread out once again and forms into the universe again. This is a neverending cycle of big bangs. The universe is pretty much refreshing itsself. In order for us to survive, we must find a way to somehow shield one part of the universe from this big bang. We will need some pretty advanced stuff (probably involving black holes), to shield ourselves. After the big bang happens again, we will be roaming around in the dark universe forever. Its pretty dark thinking about this stuff. Also, if it is impossible to escape the forces of the universe and avoid being destroyed in the big bang, we will all die and our whole existance, everything everyone has ever done, erased. D: This cycle has always been happening, so it is highly likely there was another form of life as advanced as us in the universe, and was wiped out by the heat death and then the big bang. well, thats enough pessimistic talk for one day.
Pingback: Entropy | Ben Tatman
If the universe is infinitely large, that would mean that it is not a closed system. This means that the second law of thermodynamics does not apply, and there would not be a heat death.
@Benson: “In nonrelativistic classical mechanics, a closed system is a physical system that doesn’t exchange any matter with its surroundings, and isn’t subject to any force whose source is external to the system.”
So an infinitely large universe is really an _ideal_ closed system.
Could we be like fish in the Sea? Not able to sea the water we live in? This quantum sea is an interesting sea in my mind. Like a sea that is vast and warm, ours has a current (time) and a corrosive nature (entropy/Hawking radiation). Our sea has waves (gravitational waves) and it imparts pressure. This is where I think gravity in my understanding comes in. So this big bang goes off and fuses large amounts of the otherwise homogeneous quantum sea into Baryonic matter. So this new stuff spreads out like a grenade under water. Except the nature of the quantum sea is such that instead the water pushing everything back together, the quantum sea has to use entropy to cool matter to the point where it breaks down into its base state, like a flat calm morning on a lake.
Gravity is the same regardless of density, only the rate of entropy (time dilation) changes on an object of the same mass at different densities or high gravity. Time has an energetic component and that is what dark energy is. The greater the area of open sea the faster time flows, which accounts for some extra red shift when looking through large areas of dark energy.
I am probably miles off but I would love your comments. Thanks everyone 😉
I disagree with this answer.
To me it seem obvious that as the Universe after the initial “heat death” continues on to reach zero (or possibly below – yes, it is possible) Kelvin. And due to that, the Bose-Einstein condensation takes effect, and all the atoms start to condense together into one giant wave (and stop being particles).
Then this giant singular wave vibration (which the hippies like to call “ohm”) inevitably collapses inwards and implodes (Ulrich Schneider’s observation of super cooled quantum gas) reaching singularity, the right conditions for the Big Bang.
Actually, it’s proven a myriad of wacky unforeseen things can happen at the quantum level at super low temperatures so, the Big Bang – Heat Death cycle/pulse theory is more than possible. “After heat death… they’ll happen exactly as you might expect” says the author. I’m raising the BS flag on that one.
Regarding Bose-Einstein Condensation, this has been asked many times over the years and the expert answer is always no, it won’t occur during heat death.
I’ll quote one answer from Sam Needham on Quora: “…low temperature is not enough to make a BEC. You also need bosons, and they all need to be in the same quantum ground state. If there are fermions in the universe (there are) that changes things, and the uneven distribution of matter results in different ground states for spatially separated bosons.”
there has to a replenishment big bang or contraction as a die out of galaxies is well unlogical,in all of time,yes ,before the big bang there had to mass or we would not exist now
and time has to be infinite(what existed before and before and before).why?????? hasn’t the universe already blinked out given that time is ultimately infinite.there had to something there before and a starting point is well unlogical.
@alan What is north of the north pole? There has to be something there, right? Travelling south has to be infinite. A starting point for north is unlogical.
At a certain point, wouldn’t nothing happen because each particle would be isolated by the expansion of the universe, so there isn’t an infinite amount of time for something incredibly unlikely to occur?
Mr Physicist, you are incorrect! Since it doesn’t make sense to ask what was “here before” the big bang. there is no “here before,” no “outside” to speak of, it doesn’t make sense to talk about “heat” at the “maximum entropy level” of the “Universe.” By the time uniformity is reached, all reference frames have ceased to exist, space-time has no meaning, and all of the energy that has ever existed, still exists…and it will expand and define itself.
The energy of the past Universe is Conserved as the Singularity of the next Universe.
I’m pretty sure that anything that has the even the absolutely most inconceivably tiny probability of happening by chance will eventually happen in an infinite universe over an infinite period of time. Remember, 10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^10^TREE(3) years is absolutely insignificant compared to an infinite amount of time.
Maybe we could somehow find a way to reverse entropy, or find a form of anti-entropy just like we found antimatter.
Heat death has already occurred. We exist within an improbable bubble. A universal fart, if you will.
@Louis Bevilacqua:
“I understand, perhaps incorrectly, that life for example is a low entropy state the formation of which requires energy which increase the universes overall entropy?”
Yes. Life and evolution are not violations of the second law of thermodynamics.
@MK:
“I was wondering, does the notion of time depend on entropy?”
No, it does not. Classically, many people call entropy “the arrow of time” due to the simple fact that they assume it strictly increases over time, and that we cannot go back in time, but this is really ad hoc and not well supported, as we can see in this article. Time has a very rigorous definition, and it is well-described by the theory of general relativity.
“If there’s no more source or sink for energy, then does time still even exist?”
Yes, the existence of spacetime is not dependent upon that of energy potentials.
“If the energy can fluctuate randomly though, can’t disappear, and enough time passes, then could not the remotest of all possibilities still become more than likely to happen?”
No. Probability is not dependent upon spacetime. The statement “In an infinite universe with infinite time, every possible event will happen” is sort of not exactly accurate and it contradicts the very fundamental assumptions of probability theory. So, no, the answer will always be no. The events can happen, but the probability will remain low regardless of what happens.
“Sort of like how there’s more than a 50% chance that two people in a room of 30 will have the same birthday.”
This actually is not particularly improbable, though. You are conflating the infiniteness of spacetime, which has no bearong on probability, with the ratio of the sample size to the ratio of the population size, which does have a significant effect on probability.
“I’m thinking along the lines though of all the energy gathering into a single point of order to create a source like the Big Bang 2.0.”
This is not quite how the Big Bang worked, so no.
“Or if you redefine the length of time it took for that to happen as a single smallest unit of time on some grander scale of events, then what would time passing seem like?”
Redefining the word “smallest” does not work like that. If the Planck time is, for all intents and practical purposes, the smallest, then it cannot stop being the smallest if something bigger is discovered.
“I guess the thought is, instead of a brain spontaneously emerging fully formed, that all of the structure inherent in the evolution and development of something simpler such as a cellular organism and it’s environmental pressures might be statistically more likely to spontaneously emerge. Then that could lead to the brain forming.”
Or maybe the brain will form spontaneously. It’s very clear you didn’t understand what his comment was referring to. What he meant is that any possible microstate in the universe could happen, whether this microstate form a brain or a cell or a horse or a proton or Boeing 676.
@Joe:
“Hey, but isn’t at this precise moment the Universe expanding and then it will be shrinking and when it finally shrinks there will be another Big Bang so the universe dies and borns eternally?”
Not sure what you meant, but nothing about this implies the universe will suddenly start shrinking. The universe will only start shrinking if the density parameter decreases over time such that the universe has closed shape. There is no evidence for this, though.
@Zed:
“So… expanding universe = eventual heat death via entropy.”
No, not true. Eventual heat death would occur even in a shrinking universe. The second law of thermodynamics is independent of the shape of the universe or of its size.
“But what drives that expansion?”
Dark energy and/or vacuum energy density. It is unknown if dark energy and the vacuum energy density represent the same physical object or not, but it is known it is one of the two at least.
“If it were only the laws of motion the centre of the universe could be identified – the origin point.”
There is no origin point. The universe did not originate from a single point in spacetime. Remember that spacetime itself expanded from the Big Bang. Spacetime has a size, an extent, and it was much smaller than before, but however small it was, the Big Bang happened everywhere in spacetime. There is no center of the universe. There is no possible geometry of spacetime in which such a center for the total universe could exist, except in a closed hypersphere universe, but the evidence indicates we are not in such a universe. Assuming the universe is flat, which is what measurements suggest, then there is no center of the universe.
“But as everything is moving away from everything else, that implies that other forces are at work as well.”
No, it really does not.
“Pressures from stars and the like.”
Pressures from the star push inwards, not outwards. Matter does not repel by default.
“But once those are gone, I suspect that the one active force left in the universe will start having a greater effect.”
You say this as if the other forces “will die”. This is not quite how forces work, though. to be precise, they are not forces, either, but rather quantum fields of interaction.
“Gravity. While negligible in the current greater universe, its very nature is contrary to expansion. Eventually, over a similar timeframe as the life of the universe, super gravitational forces will have a greater effect, first on their surroundings, then on each other.”
There is literally zero evidence for this. In fact, what the evidence suggests is the complete opposite. On cosmological scales, gravity is a function of 1/d^2, the reciprocal of the distance squared, and the larger d is, the “smaller” the force of gravity. Since the universe is expanding, gravity is only going to get weaker, not stronger.
“This will continue until all that exists once again is at a single point, recreating the conditions of our Big Bang.
Or I could have no idea of what I’m talking about and am just throwing out an idea.”
Probably the latter, honestly, but a noble attempt. It seems to me you were referencing the Big Crunch, but the Big Crunch is only possible under exactly on set of conditions out of possible several sets, all others more likely than this one. So, no, we can be fairly certain the Big Crunch will not happen. The evidence suggests the density parameter is not going to all of a sudden shift from being unity to being bigger than unity.
@wil mackintosh
Not quite how probability works.
@andy
“I have a theory that we are already at the end stage of the universe.”
Well, unfortunately for you, your theory is complete bunk.
“Currently the only sources of heat and energy are stars while rest of the Universe is cold and dead, but it may not have been like this in the past.”
Calculations suggest that we are at the very beginning of the stelliferous era, as 80% of stars are still near their initial phases, or at mid-stage the latest. Also, there are many sources of energy, such as radiation, decay interactions, etc. https://arxiv.org/pdf/astro-ph/9701131.pdf
@Bob
“My theory is that once all the energy is turned into heat, gravity comes into play, and all the heat is brought back to the center and get more and more intense.”
Your theory is bunk too. Firstly, let me kindly remind you that you are using the word “heat” incorrectly. Heat refers to a transfer of energy between systems, it is not a type of energy, and it is not a state function. So really, you should be saying “all the energy is brought back to the center”. However, this fixing of your vocabulary does not help your theory, since your theory still has multiple problems. The main problem is that it has no evidence whatsoever, and so, as such, it only deserves the name “hypothesis”, not “theory”. It having no evidence, it is not a good hypothesis considering there are other hypothesis that do. Secondly, your mechanism makes no sense and it very much goes against the fact that 1) the universe is expanding 2) gravity weakens over greater distances, which do occur due to the expansion 3) per the current evidence, the universe could not possibly have a center.
“Eventually, there is too much and it results in another big bang. matter is spread out once again and forms into the universe again.”
Unfortunately not quite how the Big Bang works. Matter and energy were not all concentrated into a single point in spacetime like people are imagining it. It’s more like spacetime itself was tiny, but the Big Bang occurred throughout all of spacetime, and it was only because it occurred that spacetime began to expand so quickly until it expanded to what it is today, dispersing the matter through the universe by way of expansion as opposed to it being an explosion like you’re describing it.
“In order for us to survive, we must find a way to somehow shield one part of the universe from this big bang.”
This would be literally impossible, since the Big Bang would cause temperatures larger than the Planck temperature, at which point matter itself melts on a fundamental scale.
“We will need some pretty advanced stuff (probably involving black holes), to shield ourselves.”
You cannot shield yourselves from melting matter itself. Black holes couldn’t even come close to cutting it, although I’m not even sure how you build a shield out of black holes for anything to begin with.
“After the big bang happens again, we will be roaming around in the dark universe forever.”
No evidence for this, considering that this did not happen in our universe.
“Also, if it is impossible to escape the forces of the universe and avoid being destroyed in the big bang, we will all die and our whole existance, everything everyone has ever done, erased. D:”
Correct. Not that it matters, because we’ll be long dead WAY before this happens. Protons themselves will have decayed.
“This cycle has always been happening, so it is highly likely there was another form of life as advanced as us in the universe, and was wiped out by the heat death and then the big bang. well, thats enough pessimistic talk for one day.”
It’s not pessimistic strictly because there is less than zero evidence for your hypothesis. On a hypothetical, mathematical level, its totally plausible, but when you look at actual data we have about the different constants needed to determine whether this would happen or not, it just doesn’t match it. The universe is more likely to undergo a late inflationary period that may never end and go on infinitely.
Im not disputing that you have to have energy to create. But at the same time we dont know where the energy came for the big bang. That energy could be created again from the same source and we could all replay the universe all over again.
theres an mulitaverse that theres infinite univereses we dont need to worry about ours or should we for the multiverses
I’ve always wondered, and what I read added to it somewhat. even though the universe rebooting thing could definitely happen, who to say it hasn’t already, what if this is the 2nd big bang? the existence of energy at all to even make a big bang is weird, like what came from where? how did that come to be? and also who’s to say that this isn’t the 90th big bang? the unlikely event that is our existence only happened once?
the question of WHY? will probably never get answered
thanks for coming to my TED talk
who is the publisher
@Angel Mendez-Rivera The smallest scape of fluctuations could leads to a big changes, every statememts you made is absoluly wrong here. Nothing cannot be erased forever, nothing goes forever, including the heat death of the universe.
Angel Mendez-Rivera Fluctuations are multiples, not just a single thing. Also, there are multiple types of fluctuations exist. Did you ever know about Poincaré Recurrence or Penrose’s Conformal Cyclic Cosmology? Seriously, what’s wrong with your statements? It’s like you hate this world so much that you want it to end. You really need to understamd that there is a chance that a universe can exist after heat death of the universe, and it’s not like any stupid crap imaginations. The chances of the universe to come back by fluctuations is might low but never zero.
@Angel Mendez-Rivera
“No. Probability is not dependent upon spacetime. The statement “In an infinite universe with infinite time, every possible event will happen” is sort of not exactly accurate and it contradicts the very fundamental assumptions of probability theory. So, no, the answer will always be no. The events can happen, but the probability will remain low regardless of what happens.”
That’s a very false argument right there. Probability doesn’t NEED to be dependant upon spacetime, because it could happens randomly without any orders. The probability of random events cannot be low if there are are multiple of them happens at the same time, or at least happens continuously from one to another. The statement “In an infinite universe with infinite time, every possible event will happen” never contradicts any fundamental assumption of probability theory because like I said, probability could happens randomly.
“Correct. Not that it matters, because we’ll be long dead WAY before this happens. Protons themselves will have decayed.”
You seem to forgot that there are also a scenario that protons don’t even decay at all.