Physicist:
(The following paragraph is wrong. Like, really wrong. There’s a redaction here: My bad: If atoms are mostly made up of empty space, why do things feel solid?)
As atoms get too close to one another their charges begin to repel each other. Once they’re close enough that they can “see” the other atom, the electrons on the near side of both atoms begin to repel each other and move more to the far side of both atoms. This leaves the positively charged nuclei facing each other.
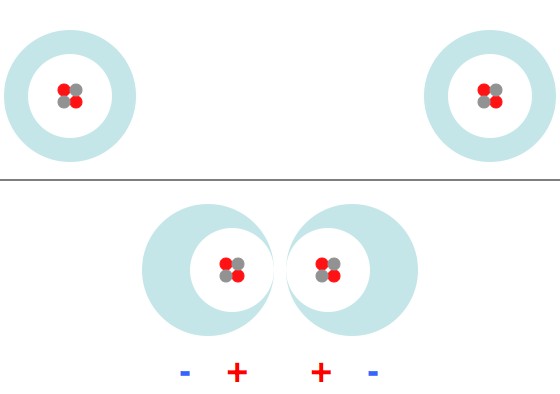
Electrons swarm around the nuclei of their atoms (in this case Helium 4). When they're brought very close together the electron clouds shift and the atoms briefly polarize in such a way that they repel.
Basically, when two atoms come too close they behave exactly like magnets brought together with the “north” ends pointing together.
(Everything up to here has been wrong.)
This certainly isn’t the whole story. Quantum chemistry isn’t rocket science, but it’s still pretty complicated. Atoms can share electrons, or their electrons can move so that they behave like attracting magnets, and a whole mess of other things. For example, attractive van der Waal forces can show up when atoms are close, but not too close. Slight fluctuations in the arrangement of electrons in one atom induces a sympathetic arrangement in nearby atoms (this is more specifically a “London dispersion force“). As a result, the atoms end up with dipoles lined up in a “+- +-” way, instead of a “-+ +-” way, like in the picture above.
In general, the force is extremely small. But it is just strong enough to hold liquid helium to itself (otherwise it would be a gas), and hold geckos to walls.

Geckos have weird feet because they have evolved to optimize the chance of random dipole interactions between the atoms in their feet and the atoms of whatever they're climbing. As a result, they can climb vetically on materials as smooth as glass. Pictured here is a gecko excited to learn that someone remembered his birthday.
Addressing the fact that matter is mostly empty space, if you really squeeze matter you’ll find that the electrical forces can no longer hold atoms apart. Basically, they find that it’s easier for the electrons and protons to fuse together and form neutrons. Once all the charges are out of the way the atoms (now balls of neutrons) are free to collapse together. At that point the only thing holding them apart is “Pauli pressure”, which is fancy quantum physics speak for “they can’t be in the same place”.
The only time this happens in nature is in neutron stars. To get an idea for what happens when you “deflate” matter: If you were to crush a 50m Olympic size swimming pool into neutron star material, it would be about 0.05mm long, which is about the width of a single hair.







Hello, I would like to know about the picture above, where the Geko learned that someone knew it is his birthday.
How many animals do you think know it is their birthday ? I think all animals should get more love and respect, so any information would be awesome.
Thank you
I also wanted to ask, is there proof if light is stretching, if it is pushed depending on amount of energy, or is there mass ?
Is speed of thought and or speed of atom faster then speed of light ?
Can love become a universal language ?
I saw a documentary on Tv about energy, it was almost jello like thick air, is it energy only or is there more, intelligence, atoms … ?
Very interesting stuff here. Have scientist made any break through at all into passing a solid through another like in the old sci-fi movie ” 4D-man” ? A resonance sort of reaction perhaps. Finding a frequency in some form that would allow this to happen at least in part. A small fusion of materials intermingling . Course 2 solids cant be in same spot at once unless maybe that “spot” became much more dense. Anyway, just wondered if any progress at all has happened in this.
If matter contain atoms then if we take a notebook,pen ,
Book or a table ,chair anything..then in sense they all are matter as they have mass and occupy space
So due to rotation of electrons in orbits of atoms of a book for example
Why we are not able to see any change in stucture of book due to movement of electrons ???
if 90% space of an atom is empty then why light can’t pass through a wall…?????
Q::–we often say that matter is made up of atoms which continuously move .Then why a ball or pen etc does not move by itself if kept stand still.?
Pingback: Evolution, The Religion Of Fools. In One Picture. | Start Thinking Right
If the body is mostly empty space what is it that is feeling the pain? Nerves are basically hollowed out tubes as is the chemicals that interacted with the nerve endings. It makes me wonder if the water feels seeing as how we are 70 -90% composed of the stuff.
I thought there were only a couple of forces – the electromagnetic, strong, weak and gravity. What is “van der Waal” force (sounds like a Dutch person) and London dispersion force? How many forces are there really?
@Daniel
They’re not new forces, just particular ways that the electromagnetic force manifests.
In an atom there is a nucleus ,revolving electrons ,protons,neutrons etc in a very small space then how 90% space of an atom is empty.
Bilal: Scientists say atoms are 99.999999999999% relatively empty space. Ones gathers electromagnetism/strong & weak nuclear forces + gravity interact via fields supporting ensemble aggragation to create something of a relativity-illusion of solidity. This may be flawed, but the 99.999999999999% (relatively) empty space is apparently a given amongst physicists who grasp such things. Astonishing to the nth degree to we common folks!!!
Every thing is made of atoms then if in a solid with simple cubic lattice arrangement of atoms at 300k , if an impulse (representing the transfer of force) just starts to travel through one face(contact force of magnitude F) the solid to produce the momentum in the 1 m cube body of the features mentioned above in space.
What is the transmission time of the force from initial face to the opposite face of the cube?? In terms of , or transmission time as a function of:F,m,T,any intrinsic initial property.
If everything is made up of matter and matter is at the end made up of waves ,then how can we hold waves???
Looking at the proton and Neutron with respect of the hadrons, Is there a possibility of interchangeability of the +ve proton to neutron rapidly/thousand of times which develop the nuclear force (Strong interaction force)
Among other reasons, the table feels solid to touch(though composed of atoms which
are mostly space) is-
(i) because the electro-magnetically charged particles of the wood repels similarly
charged particles of your palm- force of repulsion
(ii) also because of the dual nature of particles -as per Quantum mechanics ,they
function both as particles & wave.
Along the same vein of Daniel’s question, how is dark energy included with the four forces (I realize the energy is not a force)?
I think its all down to perception in the brain using the 5 senses of the body which perceive reality to be a solid, liquid or gas even though the energy is in wave form and atoms are 99.999999999999% relatively empty space.
when a photon interacts with an electron and the electron assumes a higher orbit why is it that when our bodies are bombarded by sun light or any light our bodies don’t just fly appart?
When I look into my telescope at night, I can clearly see Uranus. Is that normal? Do you feel offended?
I thought that electrons would be shared by the electrons as they revolves around them. So an electron form atom a in its trajectory would also revolves around atom b because of its proximity.